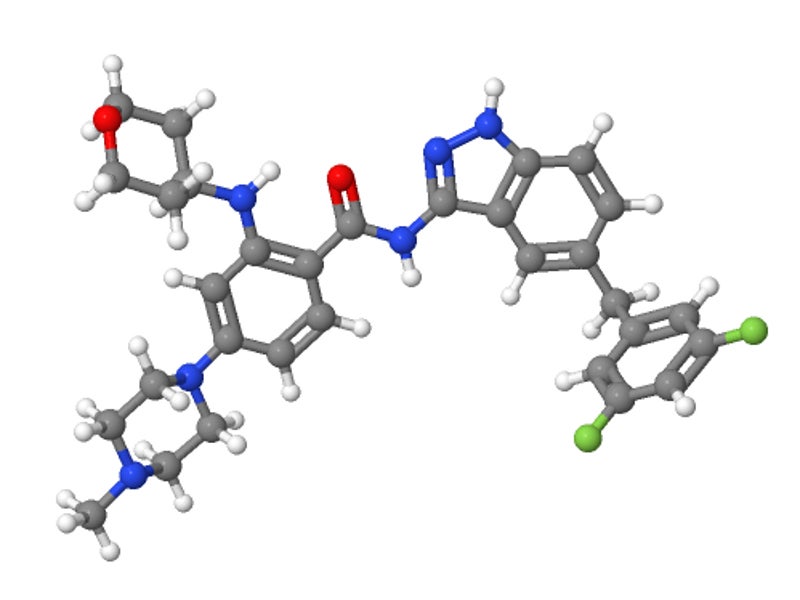
Rozlytrek (entrectinib) is a kinase inhibitor indicated for the treatment of neurotrophic tyrosine receptor kinase (NTRK) fusion-positive, advanced solid tumours in adult and paediatric patients.
Available as a hard capsule for oral administration, the drug is being developed by Roche and its Japanese subsidiary Chugai Pharmaceutical for the treatment of tumours with ROS1 or NTRK fusions. Chugai holds development and commercialisation rights to the drug in Japan, while Roche will market the drug in the rest of the world.
Rozlytrek received breakthrough therapy and orphan drug designations from the US Food and Drug Administration (FDA) and priority medicines (PRIME) designation from the European Medicines Agency (EMA) in 2017.
The drug was first approved by the Japanese Ministry of Health, Labour and Welfare (MHLW) in June 2019. It has secured orphan drug and Sakigake designations in the country.
Rozlytrek is currently being reviewed in Europe and the US for the treatment of advanced solid tumours. It is also being reviewed for the treatment of ROS1 fusion-positive lung cancer in Japan.
NTRK fusion-positive solid tumours causes and symptoms
An abnormal NTRK fusion gene is formed when the NTRK genes that encode for tropomyosin receptor kinase (TRK) proteins combine with other genes. The abnormal gene causes alteration in the TRK proteins, which activate the signalling pathways that lead to the growth of certain types of cancer cells.
NTRK gene fusion can occur in tumours present in any part of the body, including the breast, thyroid, lung, pancreas and neuroendocrine regions.
Some common symptoms of solid tumours include fatigue, bruising and bleeding from any body part, as well as fever, change in a person’s disposition and a loss of appetite.
Rozlytrek’s mechanism of action
Rozlytrek binds to and blocks NTRK, ROS1 and anaplastic lymphoma kinase (ALK) overexpression in various types of solid tumours. It results in the inhibition of the signalling pathways mediated by the genes required for cancer cell proliferation, causing tumour cell death.
Clinical studies on Rozlytrek
The MHLW approved Rozlytrek on the basis of combined positive outcomes of the STARTRK-2, STARTRK-1, ALKA-372-001 and STARTRK-NG trials in paediatric patients.
STARTRK-2 was a pivotal, open-label, multi-centre, global, Phase II clinical study that enrolled approximately 300 patients. The primary endpoint was objective response rate (ORR).
Approximately 56.9% of patients with NTRK fusion-positive, advanced solid tumours receiving Rozlytrek demonstrated a reduction in the size of ten different tumour types. The ORR in the patients receiving Rozlytrek whose tumour had advanced into the brain was 50%.
The STARTRK-NG clinical trial was an open-label, dose escalation, Phase I/II clinical study to evaluate the safety and efficacy of the drug in children and adolescent patients with solid tumours with or without NTRK, ROS1 or ALK fusions.
Out of the 28 patients evaluated in the study, complete response (CR) was observed in three patients, while a partial response (PR) was observed in nine. The duration of therapy in fusion-positive patients was 10.51 months with a time-to-response of 1.89 months.
The STARTRK-1 and ALKA-372-001 clinical studies are Phase I, open-label, multi-centre dose escalation clinical studies. The safety profile of the drug in these trials was similar to that observed in the previous studies.
The most common adverse events (AE) reported were diarrhoea, constipation, dysgeusia, fatigue, dizziness, anaemia, dyspnoea, nausea, oedema and an increase in weight and blood creatinine.