Jakafi (ruxolitinib) is an oral janus kinase (JAK) inhibitor indicated for the treatment of myelofibrosis. It was developed by Incyte Corp in collaboration with Novartis.
Jakafi obtained orphan drug designation from the European Commission and US Food and Drug Administration (FDA) for treating myelofibrosis.
Incyte submitted a new drug application to the FDA in June 2011, based on positive results from two Phase III trials. The drug was accepted for review and awarded priority designation in August 2011.
Jakafi was approved by the FDA in November 2011 and by the European Commission in August 2012. The drug is branded as Jakavi outside the US.
Jakafi (ruxolitinib) was also approved by the FDA in December 2014 for the treatment of polycythemia vera (PV) in patients who were unresponsive or intolerant of hydroxyurea.
Jakafi is the only FDA-approved drug for PV. It consistently controls hematocrit levels, reduces spleen volume and provides complete hematological remission, by targeting the critical element of the disease, JAK pathway.
Myelofibrosis
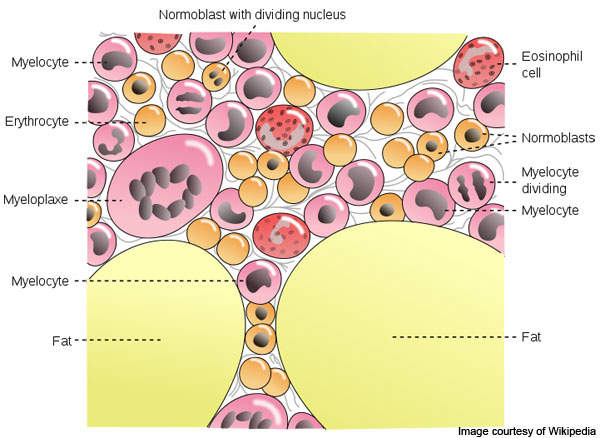
Myelofibrosis is a kind of blood cancer that affects the bone marrow. The disease restrains blood forming tissues and disrupts normal production of blood cells.
It results in extensive scarring in bone marrow, which leads to anaemia, weakness, fatigue, and often, an enlarged spleen and liver.
According to estimates in the US, the incidence of the disease is less than two in every 100,000 inhabitants. The disease generally develops in individuals over the age of 50.
Polycythemia vera (PV)
Polycythemia vera (PV) is a rare blood cancer involving overproduction of red blood cells leading to serious cardiovascular complications such as stroke and heart attack.
PV patients with inappropriate blood count levels are highly susceptible to major blood clots or cardiovascular death. It is estimated that approximately 100,000 patients in the US are suffering from PV.
Jakafi mechanism of action
Jakafi contains JAK1 and JAK2 inhibitors that are used for treating myelofibrosis. The drug works by inhibiting the signalling of cytokines (small cell-signalling protein molecules) and growth factor receptors that use JAK1 and JAK2 for signalling. The drug restrains the growth of malignant cells and also controls cytokines that contribute to hypermetabolic state.
The drug is available in the form of 10mg tablets for oral administration only.
Clinical trials
Incyte conducted Phase I clinical trials on Jakafi in May 2007.
In May 2011, a Phase II trial was initiated on myelofibrosis patients with platelet counts of 50×109/L to 100×109/L to determine the effect of the drug in improving overall survival rate and its side effects.
Recruitment started in June 2011 and the trials involved 150 patients. The primary myelofibrosis, post-essential thrombocythemia-myelofibrosis (PET-MF) and post-polycythemia vera-myelofibrosis (PPV-MF) patients were administered 5mg of ruxolitinib to study reductions in myelofibrosis associated symptoms, inflammatory cytokine and splenomegaly levels. The entire study is expected to be completed by December 2012.
Another Phase II trial was initiated in March 2011 to determine the safety and tolerability of the drug through sustained release formulation.
Approximately 40 myelofibrosis patients were recruited. The trial will evaluate the safety, efficacy and tolerability of the drug in patients with myelofibrosis, PPV-MF and PET-MF. Patients were administered once-daily doses of ruxolitinib for 16 weeks, and results will be compared with immediate release formulation. The trial is expected to be completed by January 2013.
In July 2011, Novartis Pharmaceuticals initiated a multinational, open-label Phase II trial in Asian myelofibrosis patients. Approximately 110 patients have been recruited and the entire study is expected to be completed by February 2016. Primary outcomes measured will be reduced volume in spleen, safety and tolerability of the drug.
Incyte and Novartis initiated a Phase III clinical study called RESPONSE on ruxolitinib in October 2010. The randomised study was conducted in multiple centres within and outside the US.
Incyte is responsible for the trials within the US, while Novartis is responsible for those outside the US. The trials are scheduled to be completed by July 2013.
The RESPONSE study will enrol 300 patients. The primary and secondary outcome measures of the study include finding the proportion of subjects achieving a response rate and hematologic remission respectively at week 32.
Incyte carried out the first pivotal Phase III clinical trial named COMFORT-I (COntrolled MyeloFibrosis Study with ORal JAK Inhibitor Treatment) on the drug between August 2009 and January 2011.
The randomised, double-blind and placebo-controlled study was conducted in 112 study centres across the US and enrolled 309 myelofibrosis patients. The trials were conducted under a Special Protocol Assessment agreement with the FDA.
Results of the study showed the drug met primary and secondary endpoints. The primary endpoint was attaining more than 35% reduction in spleen volume in 24 weeks.
Computerised tomography and magnetic resonance imaging techniques were used to compare response rates in the ruxolitinib-administered patient group with that of the placebo-administered group. The ruxolitinib-treated patient group achieved a response rate of 42%. The response rate in placebo-administered patients was less than 1%.
Novartis conducted the second pivotal Phase III clinical trials on ruxolitinib between July 2009 and December 2010. Named COMFORT II, the study was conducted across 56 centres in Europe and enrolled 219 patients.
Two-thirds of patients were administered with ruxolitinib and the remaining were treated with the best available therapy.
The final results of the study, announced in March 2011, demonstrated that patients who were treated with ruxolitinib achieved 35% reduction in spleen volume from baseline at week 48. The change in response rates in patients was measured by MRI or CT scans.
Marketing commentary
Incyte holds the rights to develop and market ruxolitinib in the US. Novartis obtained rights to develop and market ruxolitinib in other parts of the world excluding the US. The collaboration and license agreement was signed by the two companies in November 2009.