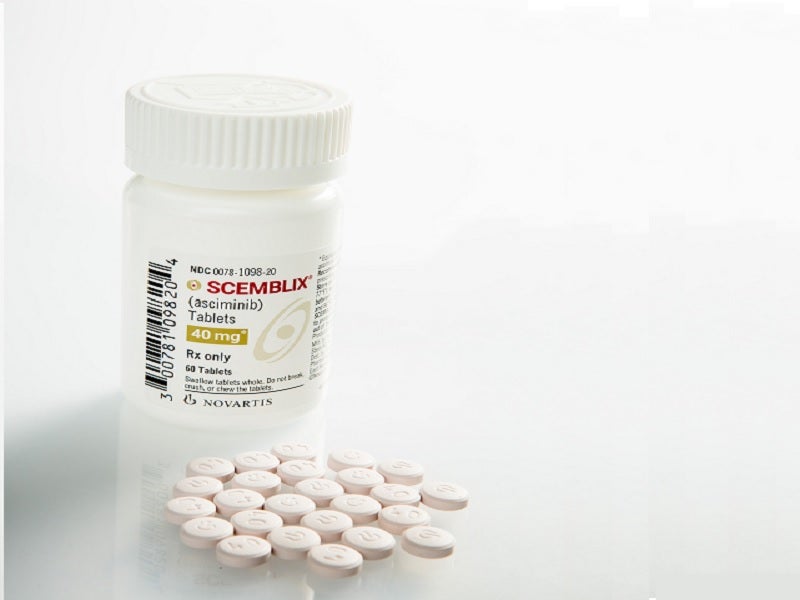
Scemblix® (asciminib) is a kinase inhibitor indicated for the treatment of chronic myeloid leukaemia (CML) in two distinct indications in adult patients.
The drug can treat patients with Philadelphia chromosome-positive CML (Ph+CML) in the chronic phase who were previously treated with more than two tyrosine kinase inhibitors (TKIs), as well as chronic phase Ph+CML patients with the T315I mutation.
Developed by Novartis, Scemblix is available as oral, unscored, round, biconvex, film-coated tablets in 20mg (pale yellow coloured) and 40mg (violet-white coloured) dosage strengths.
Regulatory approvals for Scemblix
The European Commission (EC) granted orphan designation to asciminib for the treatment of CML in March 2020.
In February 2021, Scemblix was granted two breakthrough therapy designations by the US Food and Drug Administration (FDA) for treating adult patients with Ph+CML-CP who had previously been treated with more than two TKIs and Ph+CML-CP patients with T315I mutation.
The FDA accepted and granted priority review to the new drug application (NDA) submitted by Novartis for asciminib (ABL001) in CML in August 2021.
In October 2021, the FDA granted accelerated approval to Scemblix to treat adult Ph+CML patients in CP who were previously treated with more than two TKIs and full approval to treat Ph+CML patients in CP with the T315I mutation. The drug also holds a fast-track designation from the FDA.
Chronic myeloid leukaemia (CML) causes and symptoms
CML is a bone marrow cancer that develops when an individual’s bone marrow produces too many immature white blood cells that do not function properly due to either a genetic alteration or mutation in the bone marrow’s stem cells.
The defective cells eventually build up and replace normal blood cells in the bone marrow. CML progresses more slowly than other types of leukaemia and can be of three types, namely chronic lymphocytic leukaemia, acute myeloid leukaemia and acute lymphoblastic leukaemia.
CML is a severe, life-threatening disease associated with a poor overall survival rate. It mainly affects adults and older individuals, but can also occur in children.
Symptoms of the disease at a later stage include fatigue, bone pain, loss of weight, shortness of breath, pale skin, night sweats, easy bruising and bleeding, fever, swelling, and tenderness in the left side of the belly.
Scemblix’s mechanism of action
Scemblix is an inhibitor of ABL/BCR-ABL1 tyrosine kinase. The drug has a novel mechanism of action of binding to the ABL myristoyl pocket and inhibiting the activity of the ABL1 kinase of the BCR-ABL1 fusion protein.
The drug has displayed activity against wild-type BCR-ABL1 and many mutant types of kinase, including the T315I mutation, in vitro and animal models of CML.
Scemblix, also known as a STAMP inhibitor, is being investigated in several CML-CP treatment lines, including the ASC4FIRST Phase III study, which is assessing the drug as a first-line treatment.
Clinical trials on Scemblix
Scemblix’s efficacy in treating CML has been investigated in two multi-centre and open-label clinical trials, ASCEMBL and CABL001X2101.
ASCEMBL was a randomised, active-controlled Phase III trial in which 233 Ph+CML patients in the chronic phase, who had previously been treated with two or more TKIs, were enrolled.
The patients were randomised at a 2:1 ratio to receive either 40mg of asciminib twice a day or 500mg of bosutinib once a day.
The ASCEMBL trial’s primary endpoint was the major molecular response (MMR) rate after 24 weeks. Patients treated with asciminib achieved a 25% MMR rate compared with a 13% MMR rate in patients receiving bosutinib after 24 weeks.
In the Phase I CABL001X2101 trial, 45 patients with Ph+CML in the chronic phase with the T315I mutation were administered 200mg of asciminib twice a day. The trial’s primary outcome was also the MMR rate at week 24.
The study demonstrated an MMR rate of 42% in patients by 24 weeks and 49% by 96 weeks. The median duration of treatment was 108 weeks.
The most frequent adverse reactions and laboratory abnormalities observed in patients treated with Scemblix during the trials were musculoskeletal pain, upper respiratory tract infections, rash, fatigue, diarrhoea, nausea, increased triglycerides, creatine kinase, alanine aminotransferase, lipase, amylase, decreased platelet counts, neutrophil counts, and haemoglobin.