Sotyktu™ (deucravacitinib) is an oral, allosteric tyrosine kinase 2 (TYK2) inhibitor indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for either systemic therapy or phototherapy.
Developed by biopharmaceutical company Bristol Myers Squibb (BMS), Sotyktu is currently the only approved TYK2 inhibitor in the world.
Sotyktu is available as pink-coloured round and biconvex once-daily tablets in 6mg strength. It is not indicated for use in combination with other strong immunosuppressants.
Regulatory approvals for Sotyktu
In November 2021, the US Food and Drug Administration (FDA) accepted a new drug application (NDA) for deucravacitinib for review. The FDA approved the drug in September 2022.
Sotyktu is currently under regulatory review by the European Medicines Agency (EMA) in Europe and Japan’s Ministry of Health, Labour and Welfare (MHLW).
Plaque psoriasis causes and symptoms
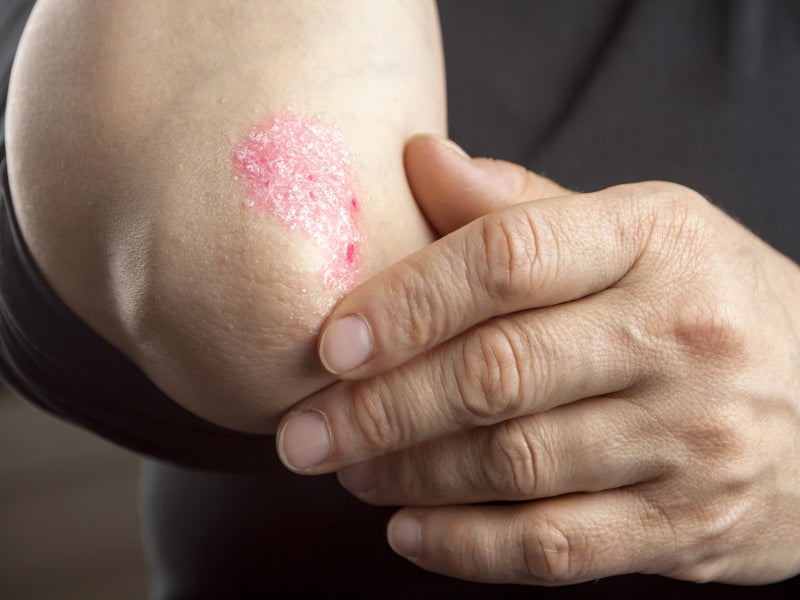
Psoriasis is a chronic, relapsing, inflammatory skin disorder characterised by abnormal epidermal keratinisation and hyperproliferation.
Plaque psoriasis causes dry, itchy, raised skin patches (plaques) covered with scales, which usually appear on the elbows, knees, lower back and scalp. The patches vary in colour depending on the patient’s skin colour.
The condition is estimated to impact 100 million people worldwide, including 14 million in Europe and 7.5 million in the US.
Sotyktu’s mechanism of action
The TYK2 signalling pathway is known to regulate several immunological mechanisms, including inflammatory cytokine signalling in IL-12 and IL-23 and type I interferons. IL-23 has been related to the development of immune-mediated disorders such as psoriasis and psoriatic arthritis.
Sotyktu (deucravacitinib) is an allosteric inhibitor of TYK2, which is a member of the Janus kinase (JAK) family. It binds to the regulatory domain of TYK2, inhibiting the interaction between the regulatory and catalytic domains of the enzyme.
The drug prevents the activation of TYK2 and subsequent downstream activation of signal transducers and activators of transcription (STATs).
The drug’s exact mechanism of action related to the inhibition of TYK2 enzyme and its therapeutic effectiveness in treating moderate-to-severe plaque psoriasis is unclear.
Clinical trials on Sotyktu
The approval of Sotyktu was based on the Phase III POETYK PSO clinical trial programme, which comprised the POETYK PSO-1 and POETYK PSO-2 clinical trials. The multi-centre, randomised, double-blind 52-week trials were designed to compare the safety and efficacy of deucravacitinib with that of placebo and Otezla (apremilast) in patients with moderate to severe plaque psoriasis.
The POETYK PSO-1 trial enrolled 666 patients, while 1,020 patients were enrolled in the POETYK PSO-2 trial.
The two studies’ primary endpoints were the percentage of patients who achieved a Psoriasis Area and Severity Index (PASI) 75 response and those who achieved a static Physician’s Global Assessment (sPGA) score of zero or one (sPGA 0/1) at week 16 compared with placebo.
Key secondary endpoints of the trials included the percentage of patients who achieved PASI 75, PASI 90 and sPGA 0/1 compared with Otezla at week 16 and 24.
In the POETYK PSO-1 trial, 58% of patients administered with deucravacitinib achieved PASI 75 and 54% achieved sPGA 0/1, compared with 13% and 7% respectively in those treated with placebo and 35% and 32% respectively in those treated with 30mg Otezla at week 16.
In the POETYK PSO-2 trial, 53% of patients treated with deucravacitinib achieved PASI 75 and 50% achieved sPGA 0/1. This was against 9% each in those treated with placebo and 40% and 34% in those treated with 30mg Otezla at week 16.
The achieved responses continued at week 52, with 80% of patients achieving PASI 75 in the POETYK PSO-1 trial and 82% of patients attaining PASI 75 in the POETYK PSO-2 trial.
The most common adverse reactions in patients treated with deucravacitinib were upper respiratory infections, increase in blood creatine phosphokinase, mouth ulcers, folliculitis, and acne.
Deucravacitinib is also being evaluated in the POETYK PSO long-term extension (LTE) trial, which is being conducted to demonstrate Sotyktu’s clinical efficacy through continuous treatment with the drug.
The two-year results of the study were released in September 2022 and demonstrated the drug’s long-term efficacy. The results analysed patients from the POETYK PSO-1 trial who had transitioned to the LTE trial. The analysis showed that 82.4% of patients achieved PASI 75, 55.2% reached PASI 90 and 66.5% attained sPGA 0/1 at 112 weeks.