Developed by Janssen Biotech, Stelara is a biologic therapy indicated for the treatment of multiple indications, including moderate to severely active Crohn’s disease, active ulcerative colitis (UC), active psoriatic arthritis, and plaque psoriasis.
The drug can be administered either subcutaneously or intravenously. The subcutaneous injection is provided in two formulations, 45mg/0.5ml and 90mg/ml, both available in single-dose prefilled syringes.
Additionally, a 45mg/0.5ml solution is offered in a single-dose vial. For intravenous infusion, the drug is supplied as a 130mg/26ml (5mg/ml) solution in a single-dose vial.
Mitsubishi Tanabe Pharma markets the drug in Japan under a copromotion agreement with Janssen Biotech.
Regulatory approvals for Stelara
In January 2019, Janssen Biotech submitted a Group Type II variation application to the European Medicines Agency (EMA) for the approval of Stelara for the treatment of adults with moderately to severely active UC.
The submission followed a supplemental Biologics License Application made to the US Food and Drug Administration (FDA) in December 2018, which also sought approval of Stelara for the treatment of adults with moderately to severely active UC.
In October 2019, the FDA approved Stelara for the treatment of adult patients with moderately to severely active UC. The approval was based on the pivotal Phase III UNIFI clinical trial, which met its primary endpoint of clinical remission.
Stelara is the first approved biologic therapy for UC. A biologics license application for Stelara was submitted to the FDA for the treatment of adult patients with moderately to severely active Crohn’s disease in November 2015, which was approved in September 2016.
The drug was approved in Europe for moderately to severely active Crohn’s disease in adults in November 2016.
Stelara was first approved in the US for the treatment of plaque psoriasis in September 2009, and active psoriatic arthritis in September 2013.
In August 2022, the FDA expanded the drug’s label to include the treatment of children six years of age and older living with active psoriatic arthritis.
The drug is also approved for plaque psoriasis in paediatric patients aged from six years to 11 years.
Crohn’s disease causes and symptoms
Crohn’s disease is a type of inflammatory bowel disease that causes inflammation and irritation in the digestive tract. It is a chronic disease mainly affecting the small intestine and the beginning of the large intestine. It begins gradually with subtle symptoms that aggravate over time.
The disease may be associated with complications such as intestinal obstruction, fistulas, abscesses, anal fissures, ulcers, malnutrition, and inflammation in other parts of the body. It can also be associated with diarrhoea, cramps and abdominal pain, weight loss, anaemia, redness in the eyes, fatigue, joint pains, nausea, and skin rashes.
The exact cause of the disease is unknown, but researchers suggest it may be caused due to an autoimmune reaction, genetics, and other factors such as smoking and the use of non-steroidal anti-inflammatory drugs.
UC is characterised as a serious, chronic, and gradual immune-arbitrated inflammatory disease of the large intestine, affecting approximately 910,000 people in the US.
Stelara’s mechanism of action
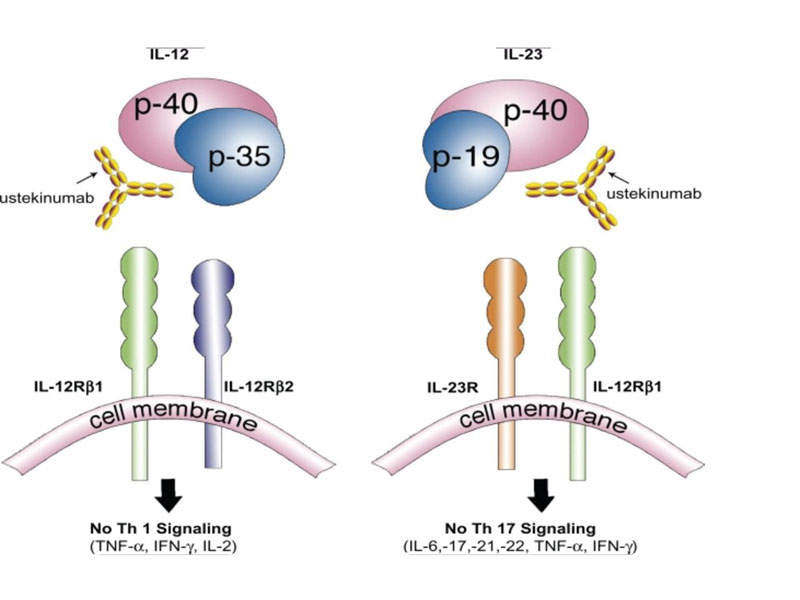
Stelara is a human IgG1K monoclonal antibody, which inhibits the mediated signals generated by interleukin (IL)-12 and IL-23 cytokines, which are associated with inflammatory and immune responses such as killer cell activation and CD4+ T-cell differentiation and activation.
The drug binds to the p40 protein subunit of the IL-12 and IL-23 cytokines to disrupt their mediated signalling and interaction with the cell surface receptor chain IL-12Rβ1.
The IL-12 and IL-23 cytokines have demonstrated an important role in inflammatory and immune responses.
Clinical trials on Stelara
The FDA and EMA approved Stelara for moderate to severely active Crohn’s disease based on results obtained from three Phase III clinical studies UNITI-1, UNITI-2 and IM-UNITI. The trials were conducted to evaluate the safety and efficacy of the drug.
UNITI-1 was a Phase III, randomised, double-blind, placebo-controlled, parallel-group, and multicentre trial conducted on 741 patients with moderate to severely active Crohn’s disease, who already received tumour necrosis factor (TNF) antagonists.
The trial subjects were randomised to receive either 130mg of Stelara as a single dose, 6mg/kg of Stelara as a single dose, or placebo as a single dose.
The trial met primary and secondary endpoints for reduction of the activity index of Crohn’s disease from baseline and clinical remission at week eight in the arms treated with Stelara 130mg and Stelara 6mg/kg when compared to the arm treated with placebo.
UNITI 2 was a Phase III, randomised, double-blind, placebo-controlled, parallel-group and multicentre trial conducted on 628 patients with moderate to severely active Crohn’s disease, who had failed conventional treatment and received TNF antagonists.
The trial subjects were randomised to receive either Stelara 130mg as a single dose, Stelara 6mg/kg as a single dose, or placebo as a single dose.
The trial arms treated with Stelara 130mg and Stelara 6mg/kg met the primary endpoints of reduction of the activity index of Crohn’s disease from baseline and secondary endpoints of clinical response and clinical remission at week eight.
IM-UNITI was a maintenance study, which enrolled 388 patients who showed positive response to a single IV dose of Stelara either in UNITI 1 or UNITI 2 studies. The patients in the trial were randomised to receive either Stelara 90mg subcutaneously every eight weeks, Stelara 90mg subcutaneously every 12 weeks, or placebo.
The trial demonstrated that the arms receiving Stelara were superior to placebo in achieving the primary and secondary endpoints.
Additional clinical trials on Stelara
The FDA approval of Stelara for treating patients with moderately to severely active UC was based on the pivotal Phase III UNIFI clinical trial.
The UNIFI clinical trials included two studies, namely the Induction study and the Maintenance study.
In the Induction study, clinical remission was attained by 19% of patients treated with Stelara within an eight-week period. Furthermore, rapid relief of symptoms was observed, with 58% of Stelara-treated patients showing a clinical response by Week 8.
In the Maintenance study, one year into treatment, 45% of patients administered with Stelara achieved remission. Furthermore, Stelara demonstrated efficacy in enabling clinical remission without the necessity of corticosteroids, with 43% of patients remaining in clinical remission and steroid-free at the one-year mark.
The primary endpoint of the study was histologic-endoscopic mucosal improvement. Histologic-endoscopic mucosal improvement is a unified measure that evaluates the improvement of the colon at the cellular level.
In the Induction study, 17% of patients receiving Stelara achieved histologic-endoscopic mucosal improvement at Week 8. In the Maintenance study, 44% of patients receiving Stelara achieved histologic-endoscopic mucosal improvement at the end of one year.