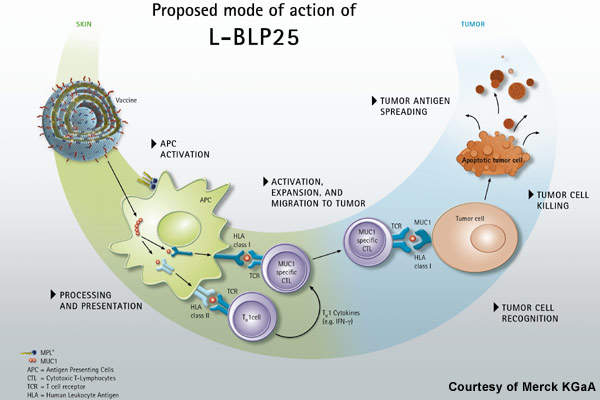
Stimuvax, formerly known as BLP25 liposome vaccine (L-BLP25), is an investigational therapeutic vaccine being developed to provide immunity against the cancer cells that over express Mucin 1 (MUC-1), a glycoprotein antigen. The synthetic MUC1 peptide vaccine is designed to identify the abnormal amount of MUC-1 on cancerous cells, control their growth and spread by stimulating the patient’s own immune system.
Stimuvax was originally developed by Cancer Research UK. The development was further continued by Oncothyreon, formerly known as Biomira. Merck is developing the drug globally under a licence agreement with Oncothyreon. The companies collaborated for the development of Stimuvax in 2001 and in December 2008 Merck acquired its inventory, manufacturing and marketing from Oncothyreon for $13m.
Merck will be responsible for the clinical development, commercial manufacturing and marketing of Stimuvax. Oncothyreon will receive royalty payments for Biologic License Application (BLA) submissions on first and second cancer indications, on regulatory approvals and on the net sales of the vaccine, when it enters the market.
Stimuvax is in three worldwide Phase III trials. The trials include START for patients suffering from advanced non-small cell lung cancer (NSCLC), STRIDE for treatment against breast cancer and INSPIRE against NSCLC, in Asian countries. INSPIRE was started on 10 December 2009.
Targeting cancer
MUC1 is an antigen commonly expressed in NSCLC and other cancers such as multiple myeloma and colorectal, breast cancer, prostate and ovarian cancers. Stimuvax consists of a 25-amino acid sequence of the MUC-1 that is expected to stimulate the T-cell mediated immune response and destroy the cancer cells. The drug is encapsulated in liposomal form to enhance the delivery towards the cancer antigen.
Stimuvax clinical trials
An early-stage trial by Merck and Oncothyreon for Stimuvax in 2004 showed positive results in 171 patients from the UK and Canada, with Stage III and IV NSCLC. Half of the patients received standard treatment for NSCLC while others were treated with Stimuvax vaccine along with standard treatment. The median survival of the patients was 30.6 months for the Stimuvax-treated patients and that of controlled treatment was 13.3 months.
Stimuvax entered Phase III clinical trials with START (Stimulating Targeted Antigenic Responses to NSCLC) in February 2007. It was the first investigational cancer vaccine to enrol patients for Phase III studies for NSCLC.
The global START trials were designed to evaluate the efficacy and safety of Stimuvax for treatment against unresectable (a tumour irremovable by surgery) Stage III NSCLC patients. The trial assessed the documented unresectable Stage III NSCLC patients, who had undergone at least two cycles of platinum based chemo-radiotherapy and demonstrated a stable disease or an objective response. It was a randomised, double-blind, placebo-controlled study.
START was designed after considerations from the European Medicines Agency (EMEA/CHMP) and was been agreed by the US Food and Drug Administration through a Special Protocol Assessment (SPA). More than 1,500 patients in about 30 countries were enrolled in the START trial.
The results of the study were announced in December 2012. The results demonstrated that Stimuvax did not meet the primary endpoint.
INSPIRE (Stimuvax trial In Asian NSCLC Patients: Stimulating Immune REsponse) is similar in design to START trials and is being conducted in China, Singapore, South Korea, Hong Kong and Taiwan. A total of 420 patients are expected to participate in the INSPIRE trials. The Phase III INSPIRE trials are also the double-blind, placebo-controlled, randomised clinical trials for the Stimuvax in unresectable Stage III NSCLC patients aimed at patient survival.
The STRIDE (STimulating immune Response In aDvanced brEast cancer) trial is an ongoing Phase III study of the Stimuvax vaccine in hormone receptor-positive, locally advanced, recurrent or metastatic breast cancer patients. The STRIDE study started in June 2009 and more than 900 patients in about 30 countries are expected to enrol in the trials. Countries from Europe, North America, Asia and Australia will participate in the trials.
The STRIDE study is being conducted in patients with progesterone receptor-positive and/or oestrogen receptor-positive, non-removable locally advanced, metastatic or recurrent breast cancer.
The investigation is conducted on patients receiving hormonal therapy by randomising it with either Stimuvax or a placebo in a 2:1 ratio. STRIDE aims at progression-free survival. The trial will assess the overall survival, tumour response, safety and quality of life.
In January 2012, Merck initiated a Phase II clinical trial of Stimuvax, known as the SPRINT study. It is a randomised, open label, and parallel assignment. It is intended to find the effects of Stimuvax in rectal cancer patients. The study enrolled 87 patients.
The primary outcome measure of the study is to find the change in tumour-infiltrating lymphocytes (TILs) evaluated by immunohistochemical analysis from the base line. The secondary outcome measures will include finding the immunological changes from baseline in the tumour microenvironment. The study results are expected to come by June 2013.
The Stimuvax Phase II study of NSCLC is ongoing in Japan.
Marketing commentary
Related project
Xalkori – Treatment for Non-Small Cell Lung Cancer
Xalkori (Crizotinib) is an oral drug indicated for the treatment of non-small cell lung cancer (NSCLC). The drug is developed and manufactured by Pfizer.
The vaccines for cancer are a new kind of treatment and are mostly unproven and are in clinical trials. Stimuvax is the first investigational therapeutic vaccine to enter Phase III studies in NSCLC and the results were disappointing. It targeted several cancers including lung cancer and breast tumours.
Merck had planned to seek approval for Stimuvax to treat lung cancer in 2012 based on the START clinical trials that began in 2007. The company estimated the sales to reach about €350m by 2017.