Symdeko™ (tezacaftor/ivacaftor and ivacaftor) is a combination drug indicated for the treatment of cystic fibrosis (CF) in people aged 12 years and above.
The drug was developed by Vertex Pharmaceuticals. The new drug application (NDA) for Symdeko™ was accepted for review by the US Food and Drug Administration (FDA) in August 2017 and it was given priority review designation. The drug was approved by the FDA in February 2018.
Vertex has also submitted a marketing authorisation application (MAA) for Symdeko™ to the European Medicines Agency (EMA). The company expects approval for the drug in Europe by the second half of 2018.
Cystic Fibrosis causes and symptoms
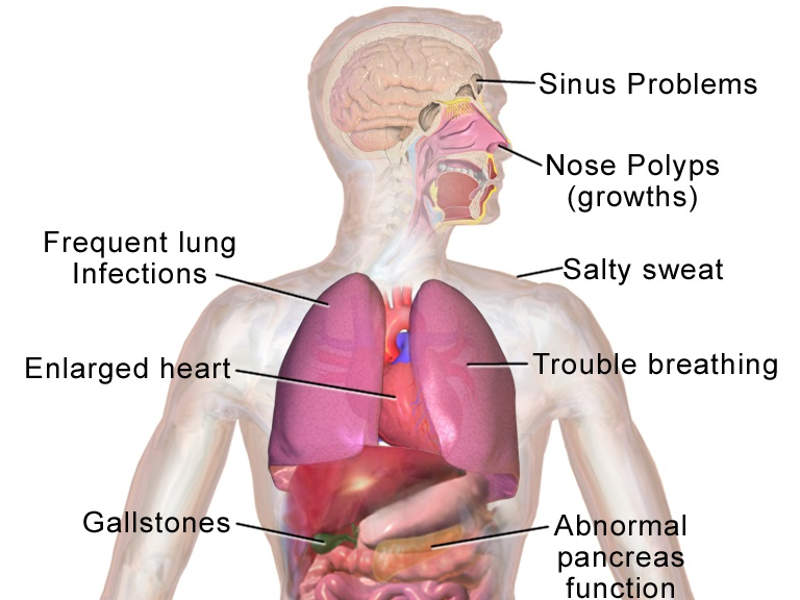
CF is a rare and life-threatening genetic disorder that occurs due to a defective or missing CF transmembrane conductance regulator (CFTR) gene. It leads to poor flow of salt and water in and out of cells.
The disease leads to the formation of abnormal, thick and sticky mucus in the lungs, which can result in lung infections and progressive lung damage.
The condition is estimated to affect 75,000 people across North America, Europe and Australia.
Symdeko’s mechanism of action
Symdeko™ contains a combination of tezacaftor and ivacaftor. Tezacaftor moves the defective CFTR protein onto the cell surface, while ivacaftor helps to facilitate the opening of the chloride channel on the cell surface to increase chloride transport.
The drug is available in 150mg dose tablets for oral administration.
Clinical trials on Symdeko
The FDA’s approval of Symdeko™ was based on results obtained from two Phase III clinical studies named EVOLVE and EXPAND. The studies enrolled 750 CF patients aged 12 years and above with either two copies of the F508del mutation or with one F508del mutation and one additional mutation that results in residual CFTR function.
The clinical programme was sponsored by Vertex Pharmaceuticals and partially funded by the Cystic Fibrosis Foundation.
The results demonstrated that patients treated with Symdeko™ achieved significant improvements in lung function.
In addition, the safety and tolerability profiles in both clinical studies were sustained up to 48 weeks.
The overall safety profile was obtained from the results of three Phase III, double-blind, placebo-controlled trials, including two parallel group trials lasting 12 and 24 weeks, respectively, and one cross-over design trial of eight weeks duration.
One Phase III clinical trial enrolled 496 CF patients aged 12 years and above. All the patients enrolled in the three studies were administered with at least one dose of Symdeko™.
Results from these studies showed that the proportion of patients that discontinued the study in Symdeko™ group due to adverse events was 1.6%, compared to 2% in placebo-treated patients.
The drug’s safety profile was similar across all subgroups of patients, including when analysed by age, sex, baseline percent predicted FEV1 (ppFEV1), and geographic regions.
The most common adverse events reported in patients administered with Symdeko™ were infective pulmonary exacerbation and cough.
Another Phase III clinical trial is currently ongoing in children aged six to 11 years with two copies of the F508del mutation or one copy of the F508del mutation and an additional second mutation.
Marketing commentary on Vertex Pharmaceuticals
Based in Boston, US, Vertex Pharmaceuticals is a biotechnology company involved in the innovation of transformative medicines for people with serious and life-threatening diseases.
The company is carrying out several research and development (R&D) programmes focused on CF and other serious diseases.
It has commercial offices located in the US, Europe, Canada, and Australia.