Tanzeum (albiglutide), developed by GlaxoSmithKline (GSK), is a GLP-1 receptor agonist indicated for treatment of type 2 diabetes. GSK received approval for Tanzeum from the US Food and Drug Administration (FDA) as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in April 2014.
Albiglutide received marketing authorisation from the European Medicines Agency (EMA) for treatment of type 2 diabetes in adult patients in March 2014. The drug is sold under the brand name Eperzan across Europe.
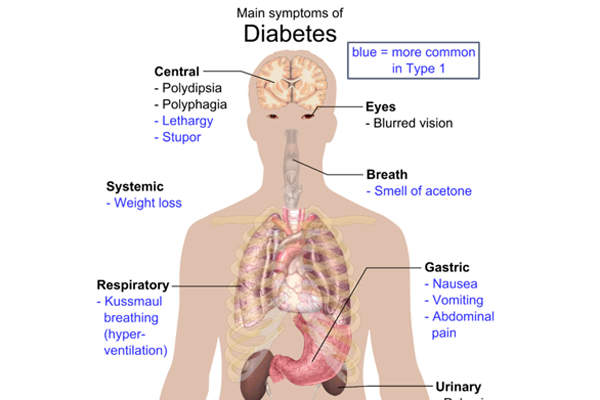
Type 2 diabetes symptoms, causes and severity
Indicated to improve glycemic control in adults with type 2 diabetes, along with diet and exercise.
Type 2 diabetes is a life-long, progressive metabolic disorder that is characterised by high levels of sugar in the blood. The symptoms of the disease include frequent urination, constant appetite and excess thirst. These symptoms are found in about 85% to 95% of the adult patients diagnosed with diabetes.
Factors that may contribute to the development of type 2 diabetes include lack of physical activity, obesity, increasing age, high blood pressure, and genetics.
Type 2 diabetics account for about 95% of the estimated 382 million diabetics worldwide and 20 million in the US.
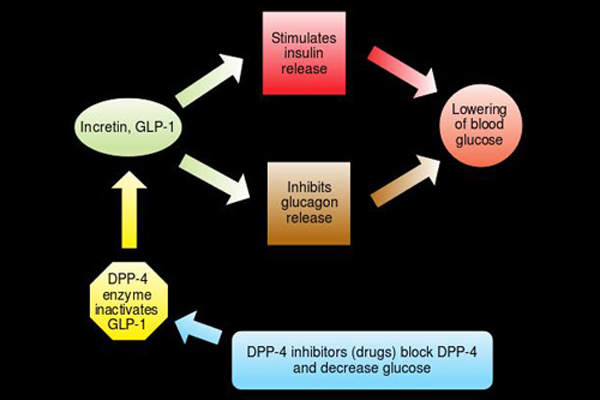
Tanzeum’s mechanism of action
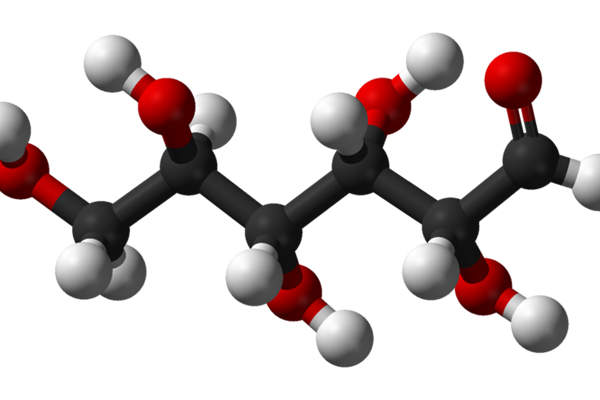
Tanzeum contains Glucagon-like peptide-1 (GLP-1) receptor agonist, which is a recombinant protein made by combining two tandem copies of modified human GLP-1 genetically merged in tandem with human albumin. The drug lowers fasting glucose and postprandial glucose excursions in type 2 diabetics. It is available in 50mg dose that can be administered through injection for subcutaneous (SC) use.
Clinical trials of Tanzeum
FDA approval for Tanzeum was based on results obtained from comprehensive Phase III study known as Harmony programme that consisted of eight clinical trials, which enrolled more than 5,000 patients and treated more than 2,000 of them with Tanzeum. The clinical studies evaluated Tanzeum against commonly used classes of type 2 diabetes treatments including insulin, metformin, glimepiride and pioglitazone.
Results showed that patients treated with Tanzeum produced clinically relevant reduction from baseline in HbA1c compared with placebo, and no overall differences in glycemic effectiveness or body weight were observed across the demographic subgroups.
The efficacy of Tanzeum was evaluated in a randomised, double-blind, placebo-controlled, multicentre phase III clinical trial that enrolled 296 type 2 diabetics. The patients were randomised to receive Tanzeum 30mg SC once weekly, Tanzeum 30mg SC once weekly uptitrated to 50mg once weekly at Week 12, or placebo.
The study results demonstrated that the patients who were treated with Tanzeum 30mg or 50mg showed statistically significant reductions in HbA1c from baseline at Week 52. It also showed that the adjusted mean change in weight from baseline did not differ significantly between Tanzeum and placebo at Week 52.
The adverse affects found in the Tanzeum group during the clinical study included upper respiratory tract infection, diarrhoea, nausea, and reaction at the injection site.
Marketing commentary of Tanzeum
GSK plans to launch Tanzeum in to the US market in the third quarter of 2014. Other medications available for the treatment of type 2 diabetes include Forxiga (dapagliflozin) developed by AstraZeneca and Bristol-Myers Squibb, and Invokana (canagliflozin) manufactured by Janssen Pharmaceuticals and Mitsubishi Tanabe Pharma Corporation.