Taranabant is a highly selective cannabinoid-1 (CB1) receptor inverse agonist developed by Merck & Co for the treatment of obesity. The Phase III taranabant study involved about 2,400 patients and was to be conducted for two years. In March 2008, after completion of 52 weeks of the study, Merck reported positive results of the drug in conjunction with diet and exercise in obese patients.
The patients experienced double the amount of weight loss by taking 2mg of taranabant when compared to the patients treated with placebo. However, in October 2008, the company discontinued the Phase III programme and clinical development of taranabant because of its side effects.
The drug showed gastrointestinal and psychiatric side effects such as increased anxiety, depression and irritability. Merck had previously planned to file for regulatory approval with the US Food and Drug Administration in 2008, but subsequently withdrew it.
Risks associated with obesity
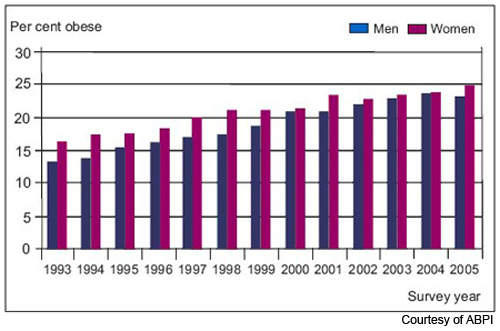
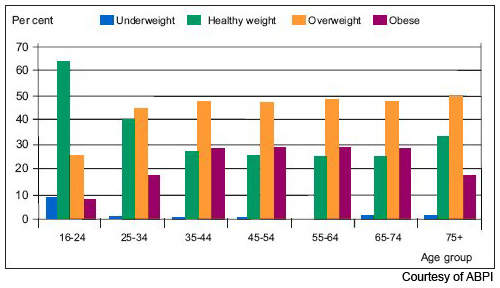
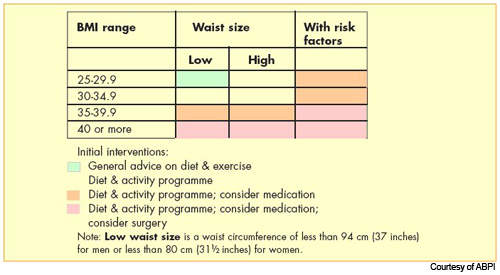
Obesity is now the most common nutritional disorder in western industrialised countries. Defined as a body mass index (BMI) of greater than 30, it arises from the accumulation of excess fat in the body from over-consumption of fatty foods. Prevalence of obesity in the US and Europe has reached epidemic levels.
Data from the World Health Organisation’s MONICA project shows that in some parts of Europe, over 70% of men aged 55–64 years are clinically obese or overweight (BMI > 25) and almost 70% of women in this age group. One in five of all Americans is obese and one in three overweight. Furthermore, increasing rates of childhood obesity are likely to exacerbate the trend towards increasing obesity in adulthood.
There is a strong association between obesity and increased risk of cardiovascular disease and diabetes, and possibly certain cancers, such as breast and colorectal cancer. The dramatic rise in the incidence of type 2 diabetes is due largely to the increased prevalence of obesity. Increases in body weight lead to changes in blood lipid and cholesterol levels, predisposing to increased risk of atherosclerosis.
Not surprisingly, the growing prevalence of obesity has stimulated the search for drugs to treat this condition.
Targeting cannabinoid receptors
The development of drugs that target cannabinoid receptors in the brain owes much to the observation that cannabis smokers often experience extreme hunger pangs, which cannabis smokers refer to as ‘the munchies’. If cannabinoids can stimulate appetite, then, in theory, targeting cannabinoid receptors might reduce appetite and consequently aid weight loss.
The central cannabinoid (CB1) receptors are believed to play a role in controlling food consumption and the phenomena of dependence/habituation and are therefore an important target for anti-obesity medications.
Merck & Co’s taranabant has a different mode of action to rimonabant, another anti-obesity medication that also targets the endocannabinoid system. Whereas taranabant acts as a selective cannabinoid-1 (CB1) receptor inverse agonist, binding to CB1 receptors, rimonabant is a CB1 receptor antagonist. It works by blocking endogenous cannabinoid binding to neuronal CB1 receptors.
Both modes of action appear to induce significant weight loss, suggesting that the endocannabinoid system is a worthwhile therapeutic target for obesity.
Early trials
Data from an earlier Phase II trial indicated that treatment with taranabant results in significant weight loss in obese patients. In this 533-patient study, 12-weeks treatment with taranabant was significantly more effective than placebo in reducing body weight as well as waist circumference.
Efficacy and safety in long-term use is an important feature of any anti-obesity drug, including those targeting the endocannabinoid system. Indeed, concerns about the safety of rimonabant, especially in relation to psychiatric side effects, had delayed the drug’s approval in the US.
Marketing commentary
The market for weight-reducing drugs has had a somewhat chequered history, characterised by major product withdrawals. Although the statistics suggest a market with enormous opportunity, pharmaceutical companies have so far been unable to capitalise on the need for anti-obesity agents.
Some potential anti-obesity medications have proved effective in the first six months of treatment, only to lose effectiveness as subjects develop resistance to treatment.
Side effects have been a major concern with all currently available anti-obesity medications, including rimonabant, which has been marketed in Europe since 2006. Clearly, the safety and tolerability profile of taranabant would have been a major factor governing regulatory approval in the US and Europe.