Tarceva (erlotinib) is an oral anti-cancer drug developed by OSI Pharmaceuticals, Genentech and Roche. It is a member of the Epidermal Growth Factor Receptor (EGFR) inhibitor class of agents and currently indicated for treatment of Non-Small Cell Lung Cancer (NSCLC) and pancreatic cancer.
Tarceva received US Food and Drug Administration (FDA) approval for the treatment of NSCLC in 2004 and gained the distinction of being the first EGFR inhibitor to show a survival benefit in lung cancer patients. European approval for treatment of NSCLC in patients failing prior chemotherapy followed in 2005. Japanese approval for the same was obtained in 2007.
On the back of successful Phase III trials in pancreatic cancer, Tarceva secured approval for treatment of advanced pancreatic cancer in combination with gemcitabine in chemotherapy-naïve patients in both the US and Europe. As the first new pancreatic cancer therapy for a decade, this represented a major development for this difficult-to-treat disease.
Tarceva was approved by the FDA as a maintenance therapy for advanced NSCLC in April 2010. The approval was based on positive results from the Phase III trial, SATURN.
Generic version leads to competition
Indian pharmaceutical firm Cipla has developed a generic version of erlotonib which is priced at a third the price of Tarceva. A tough competition emerged for Roche’s Tarceva when Cipla won the case over Roche in April 2009, and was allowed to manufacture and sell the generic version in the Indian market. Tarceva was launched in India in 2006.
Novel mode of action via inhibition of EGFR
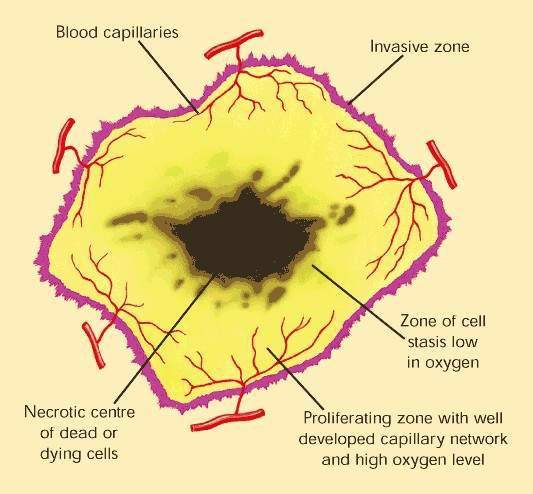
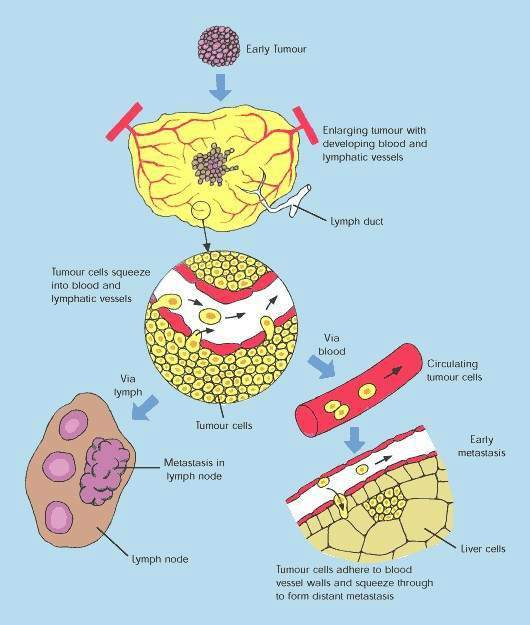
Over-expression of EGFR is common in several solid tumours, such as colorectal and lung carcinomas, and cancers of the head and neck. It correlates with increased metastasis, decreased survival and a poor prognosis. EGFR protects malignant tumour cells from the cytotoxic effects of chemotherapy and radiotherapy, making these treatments less effective.
Tarceva inhibits receptor tyrosine kinase activity, the EGFR gene’s protein product.
By interfering with cell signalling pathways involved in cell proliferation, inhibition of EGFR-associated tyrosine kinase represents a novel approach to the treatment of solid tumours. Tarceva is one of several cancer drugs that target EGFR.
Survival benefit in relapsed lung cancer patients
Cancers of the lung are of two types: NSCLC and small-cell lung cancer (SCLC). NSCLC is the most common, accounting for around 80% of all lung cancers. It is an aggressive disease, for which overall five-year survival rates are less than 10%. New forms of treatment are urgently needed for this intractable form of cancer.
Tarceva was explored as a treatment for NSCLC in a series of Phase III clinical trials in which it was used either alone or in combination with other anti-cancer agents. These trials followed promising results in Phase II studies, which showed a clear survival advantage when Tarceva was used as monotherapy in patients with chemo-refractory NSCLC.
Of the 57 evaluable patients, 51% achieved disease stabilisation and 40% survived for at least 12 months.
In a Phase II trial, patients previously treated with advanced NSCLC were administered Tarceva in combination with MetMAb. The results showed that patients with higher amounts of Met in their tumours lived twice longer and demonstrated longer progression-free survival compared to patients given Tarceva alone.
The study also revealed that MetMAb in combination with Tarceva tripled the survival in patients with advanced NSCLC. A Phase III study to further investigate MetMAb as a personalised medicine for lung cancer patients will start by the fourth quarter of 2011.
Survival benefit was subsequently confirmed in the primary registration trial, a Phase III randomised, double-blind study in which Tarceva was compared with placebo in 731 patients with NSCLC who had failed prior chemotherapy. Among patients treated with Tarceva there was a 42% improvement in median survival and a 45% improvement in one-year survival. Statistically significant improvements were also seen in all secondary endpoints, which included time to symptom deterioration, progression-free survival and response rate.
In May 2009 Roche claimed that its Phase III study ‘ATLAS’ on using Avastin with Tarceva has indicated a longer life in lung cancer patients.
These positive findings were encouraging as previous Phase III studies in which Tarceva was used as first-line therapy in combination with cytotoxic drugs (the TRIBUTE and TALENT studies) had failed to demonstrate a survival advantage when Tarceva was added to conventional treatment regimens.
In the Phase SATURN III trial, 889 patients were enrolled across 160 sites worldwide. The patients were initially treated with four cycles of platinum-based chemotherapy and later given Tarceva or placebo if the cancer did not spread. An improved progression-free survival was observed and the primary end point was met.
The European randomised trial of Tarceva versus Chemotherapy (EURTAC) was conducted in patients with advanced NSCLC. The study was designed to compare the effectiveness of Tarceva with that of the platinum-based chemotherapy in a western population. The study met its primary endpoint of progression-free survival, ahead of schedule. Tarceva was also considered to be superior to the platinum based chemotherapy in enhancing the progression-free survival.
A Phase II study named OPTIMAL, similar to the EURTAC, was carried out for an Asian population.
Tarceva appears generally well tolerated. Only rash and diarrhoea occurred with greater frequency in the Tarceva arm of the primary registration trial in comparison to placebo, a finding consistent with the other trials. Rash is a common side effect of treatment with EGFR-inhibitors and affected 75% of patients in the Tarceva NSCLC primary registration trial. It has been suggested that rash may serve as a biomarker of potential drug activity.
Tarceva confers survival benefit in pancreatic cancer
Because many solid tumours over express EGFR, Tarceva has therapeutic potential in the treatment of cancers other than just NSCLC.
Echoing the success of trials in NSCLC patients, the companies released promising data in September 2004 from a phase III trial in pancreatic cancer. Like lung cancer, pancreatic cancer has proved notoriously hard to treat and has an especially poor prognosis. Results of the 450-patient pancreatic cancer trial showed that when administered in combination with gemcitabine, Tarceva improved survival (primary endpoint).
Combination therapy produced a statistically significant 23.5% improvement in overall survival in patients with locally advanced or metastatic pancreatic cancer compared with gemcitabine alone. Median and one-year survival in the combination treatment arm were 6.4 months and 25.6% respectively, which compared with 5.9 months and 19.7% respectively in those receiving gemcitabine plus placebo. Progression-free survival was also statistically significantly greater in the combination treatment arm.
Consistent with the other clinical trials rash and diarrhoea were the main treatment-related side effects associated with administration of Tarceva. The results in pancreatic cancer showed importantly that Tarceva had efficacy beyond NSCLC, its first indication.
Other indications in which Tarceva has produced objective evidence of anti-tumour activity in patients failing standard chemotherapy include ovarian cancer as well as cancers of the head and neck.
Marketing commentary
Approval of AstraZeneca’s Iressa (gefitinib) for treatment of NSCLC provided a boost to the market prospects of EGFR inhibitors, which are seen as an important new class of anti-cancer agents. Many of the cancers in which EGFR inhibitors are being explored as potential treatment represent difficult-to-treat indications where rates of chemo-resistance and mortality are very high. Cancers of the lung and pancreas in particular represent a large and under-penetrated market, for which drugs such as Tarceva will find a ready place.