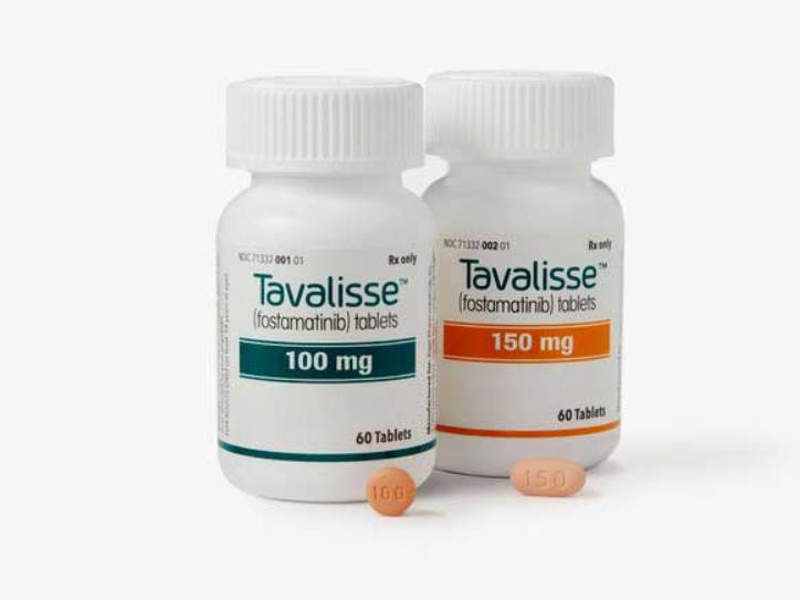
TAVALISSE™ is a twice-daily regimen indicated for the treatment of chronic immune thrombocytopenia (ITP) in adult patients who have had an insufficient response to a previous treatment.
The drug was discovered and developed by Rigel Pharmaceuticals and is an oral spleen tyrosine kinase (SYK) inhibitor.
The US Food and Drug Administration (FDA) originally granted orphan drug designation to the drug in September 2015.
Rigel Pharmaceuticals submitted a new drug application (NDA) for TAVALISSE to the US FDA in April 2017 and received approval in April 2018.
TAVALISSE is expected to be launched in the US in May 2018, while the European marketing authorisation application (MAA) is currently planned to be filed in the fourth quarter of 2018.
Chronic immune thrombocytopenia (ITP) causes and symptoms
ITP is caused by an autoimmune response, while patients with chronic ITP experience attack on their immune system that results in the destruction of the body’s own blood platelets.
ITP may be linked to viral infections such as HIV and hepatitis C in some patients.
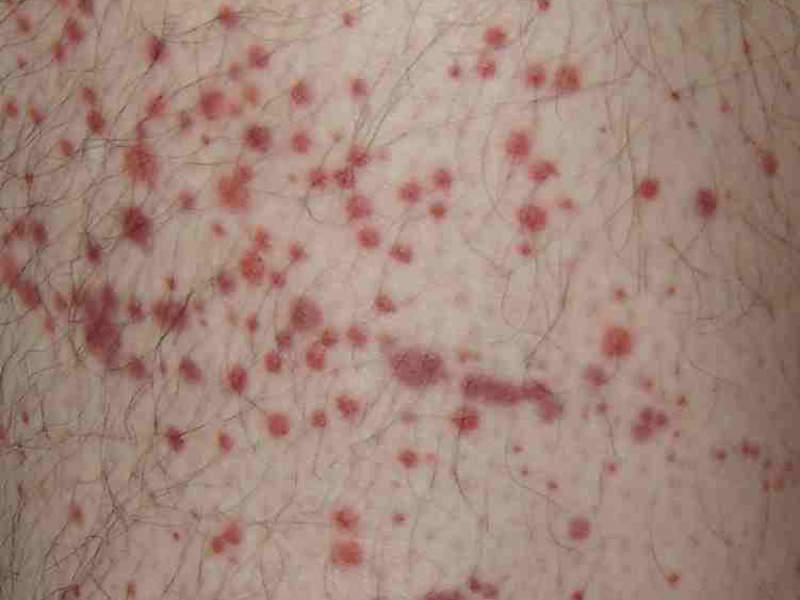
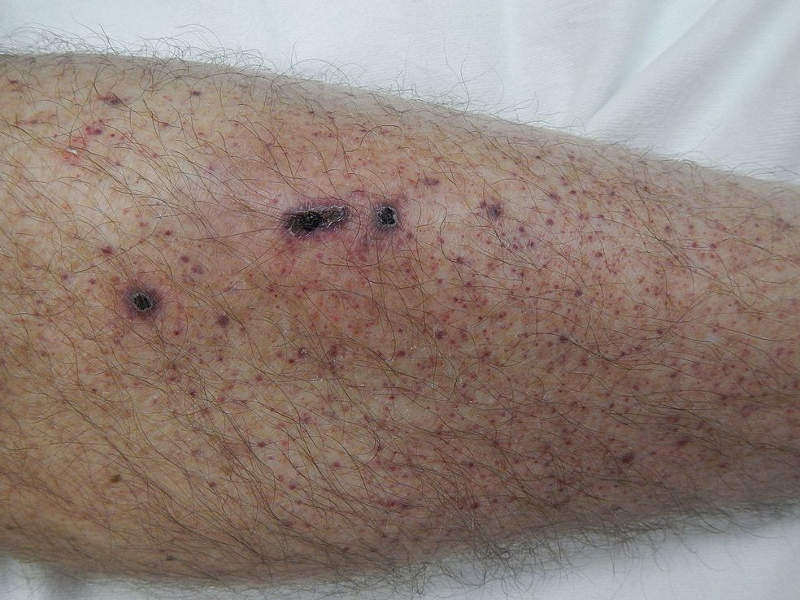
Patients with ITP also suffer from unusual bruising, bleeding and fatigue due to low platelet counts.
More than 40,000 people are affected by chronic immune thrombocytopenia (ITP) in the US.
Treatments currently available for the condition include steroids, blood platelet production boosters (TPOs) and splenectomy therapies.
TAVALISSE’s mechanism of action
TAVALISSE contains fostamatinib, an inhibitor of spleen tyrosine kinase (SYK), a key signalling component in the body’s immune process that leads to platelet destruction in ITP patients.
Fostamatinib’s major active metabolite R406 prevents signal transduction of Fc-activating receptors and the B-cell receptor.
It also reduces the antibody-mediated destruction of platelets.
The drug is available for oral use in tablet form in 100mg and 150mg doses.
Clinical trials on TAVALISSE
The FDA approval for TAVALISSE was based on data gathered from a clinical programme named FIT, which included two randomised placebo-controlled phase three trials, namely FIT-1 and FIT-2 and an open-label extension study known as FIT-3.
The approval was also based on an initial proof-of-concept study.
A total of 150 patients with a median age of 54 years participated in the FIT-1 and FIT-2 trials, also referred to as studies 047 and 048.
A total of 76 patients were enrolled in the FIT-1 study, comprising 51 that were randomised to the TAVALISSE group and 25 to the placebo group.
The FIT-2 trial involved a total of 74 patients, including 50 that were randomised to receive TAVALISSE, while the remaining participants were given the placebo.
Patients received either TAVALISSE twice daily or a matching placebo accordingly for a period of 24 weeks.
The dosage in the TAVALISSE arm was initially set at 100mg and later increased to 150mg, based on platelet count and tolerability.
The patients who did not respond to treatment after 12 weeks were eligible to enrol in the open-label extension study FIT-3.
FIT-3 saw participation from a total of 123 patients, including 44 that were previously randomised to receive the placebo and 79 patients that were previously randomised to TAVALISSE.
Side effects
The most common adverse reactions reported in the clinical studies were nausea, dizziness, diarrhoea, respiratory infection, rash, chest pain, neutropenia and hypertension.
Severe adverse drug reactions included dyspnoea and hypertension, neutropenia, arthralgia, toothache, syncope and nephrolithiasis.
Marketing commentary on Rigel Pharmaceuticals
US-based Rigel Pharmaceuticals is a clinical-stage biotechnology company that is primarily engaged in the discovery and development of novel, small-molecule drugs that are intended to improve the lives of patients with immune and hematologic disorders.
The company’s ongoing clinical trial programmes include phase two studies on fostamatinib in autoimmune haemolytic anaemia and IgA nephropathy.
In addition, Rigel is also co-developing novel product candidates with partners BerGenBio, Aclaris Therapeutics and Daiichi Sankyo.