The product of a co-development programme between ThromboGenics, a Belgian biotechnology company, and BioInvent International, TB-402 is a novel long-acting anticoagulant. It is being developed for prevention and treatment of venous thromboembolism (VTE) as well as for stroke prevention in patients with atrial fibrillation (AF).
VTE is the formation of a blood clot or thrombus in the vein, which may occlude the vein or rupture and lodge elsewhere in the body, such as an artery.
VTE encompasses deep-vein thrombosis (DVT) and pulmonary embolism (PE), in which clots formed in the deep veins of the leg rupture and become lodged in the pulmonary artery. In the US alone, it is estimated that two million people are affected by VTE, of whom about 100,000 die.
TB-402 is still in the early stages of clinical development. Having successfully completed phase I safety and tolerability studies in healthy volunteers, it has now advanced to phase II trials in patients undergoing orthopaedic surgery.
TB-402 targets coagulation factor VIII
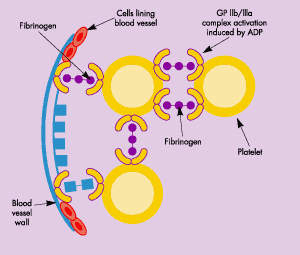
The formation of a blood clot is the end product of a highly regulated enzymatic cascade process called the coagulation cascade. ThromboGenics’ TB-402 targets Factor VIII (antihaemophilic factor), an essential blood clotting factor that acts as co-factor for Factor IXa in the activation of Factor X.
TB-402 is a recombinant human monoclonal antibody (MAb) that binds with high affinity to the C1 domain of Factor VIII, partially inhibiting the action of Factor VIII. Acting as a partial Factor VIII inhibitor, TB-402 is designed to prevent thrombosis while maintaining haemostasis.
Advances in anticoagulation therapy
Standard anticoagulant therapies for the prevention of VTE include low molecular weight heparins, pentasaccharides and vitamin K antagonists, all of which have inherent limitations.
In clinical use for over 50 years, the vitamin K antagonist warfarin remains the standard long-term oral anticoagulant. Although effective when well managed, it has a number of significant drawbacks that can have potential thrombotic and haemorrhagic consequences.
Warfarin is an indirect thrombin inhibitor and depletes vitamin K-dependent clotting factors, particularly prothrombin, when exerting its antithrombotic effect.
Because of its slow onset of action, patients have to take overlapping anti-thrombin therapy with injectable heparins when rapid anticoagulation is indicated.
More recently the market has seen the introduction of new oral anticoagulants in the form of a direct thrombin inhibitor (dabigatran etexilate) and a direct Factor Xa inhibitor (rivaroxaban).
Although ThromboGenics’ TB-402 is an injectable anticoagulant, its long half-life may permit reduced dosing compared with existing injectable anticoagulants, which have to be administered daily. It is envisaged that only a single postoperative injection will be needed for treating DVT and a monthly injection for stroke prevention in AF patients.
TB-402 progresses in clinical trials
A phase I clinical trial in 56 healthy volunteers showed that TB-402 met its primary safety and tolerability endpoints as well as secondary pharmacokinetic and pharmacodynamic endpoints. No serious adverse events emerged across the dose range tested (up to 1.8mg/kg). There were also no major bleeding complications or increased minor bleeding events compared with placebo.
A Phase II clinical trial of TB-402 was initiated in December 2008, recruiting 316 patients across 30 sites in Europe. The randomised, dope-escalating, multicentre study investigated TB-402 in comparison with enoxaparin in preventing VTE in patients who have undergone knee replacement surgery.
In July 2010, BioInvent and ThromboGenics presented positive results from the Phase II trial at the 21st International Congress on Thrombosis in Milan, Italy. The results showed that TB-402 was more effective in preventing VTE when compared to enoxaparin.
In April 2011, a phase IIb trial was initiated to determine the safety and efficacy of the drug in combination with rivaroxaban in patients who have undergone hip surgery. Around 600 patients across 41 European countries have been enrolled on the study. The first patient was dosed in April 2011. The trial is scheduled to be completed by July 2012.
Marketing commentary
After heart attack and stroke, VTE is the third most common cardiovascular disease. In the majority of cases VTE is a silent disease, producing no overt symptoms and as a consequence under-diagnosed.
Patients undergoing major orthopaedic surgery (hip or knee replacements and hip fracture) are at an especially high risk of developing VTE. Without anticoagulant therapy, between 40 and 50% of patients undergoing hip replacement surgery suffer VTE. This rises to 70–80% in hip fracture surgery.
With potential for single-dose treatment of patients undergoing orthopaedic surgery, TB-402 could offer an attractive alternative to injectable low molecular weight heparins, such as enoxaparin.
Partial inhibition of Factor VIII activity may reduce the risk of increased bleeding, a known side-effect of anticoagulants, and the need for regular blood coagulation monitoring.