TBRIA (calcitonin-salmon [rDNA origin] delayed release tablet) is the first oral recombinant salmon calcitonin, developed by Tarsa Therapeutics, for the treatment of postmenopausal osteoporosis.
Tarsa Therapeutics submitted a new drug application (NDA) for TBRIA for the treatment of postmenopausal osteoporosis in women with at least five years postmenopause to US Food and Drug Administration (FDA) in July 2015.
The FDA accepted the NDA for the review and fixed the approval deadline date as 30 May 2016.
Causes and severity of postmenopausal osteoporosis
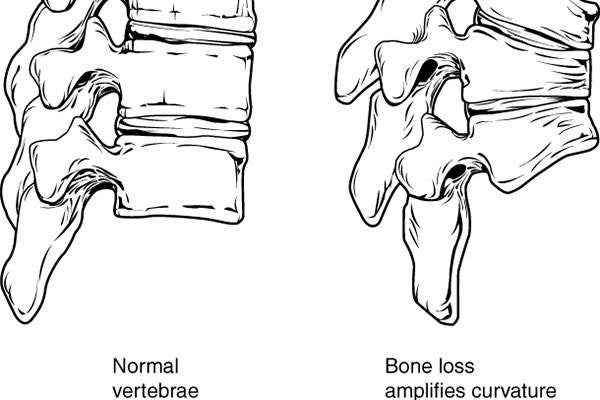
Osteoporosis is a common condition of thin bone with an increased risk to fracture. The major factors for developing osteoporosis include age, family history, postmenopausal status, lower calcium intake and use of drugs such as corticosteroids.
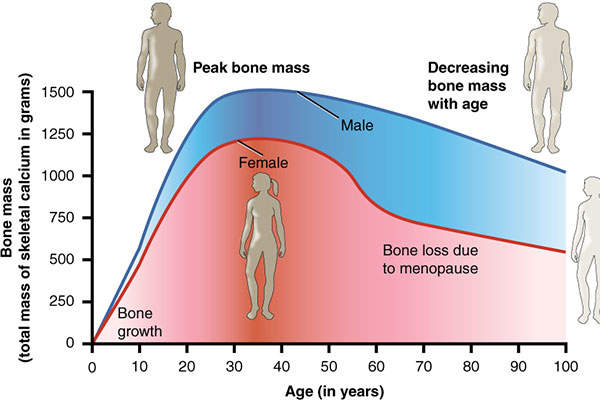
Postmenopausal osteoporosis is a condition in which the oestrogen hormone affects bone health with the onset of menopause. It is prevalent in women over the age of 50.
According to the National Osteoporosis Foundation estimates, approximately ten million people in the US and 65 million people in Europe and Japan are suffering from osteoporosis, while approximately 34 million people have osteopenia (low bone mass).
Mechanism of action of TBRIA
TBRIA is an oral formulation of salmon calcitonin, which is more potent than the human calcitonin hormone. Salmon calcitonin is a peptide hormone that inhibits bone resorption, which is the underlying cause for the development of osteoporosis.
The drug, without undergoing any degradation, moves across the intestinal lining, enters the blood stream through the stomach and binds with bone cells at a receptor known as osteoclasts, and stops and prevents bone breakdown process and loss of bone mass.
The drug will be available in the form of a tablet for oral administration only.
Clinical trials on TBRIA
Actonel is one of the most widely used osteoporosis drugs on the market.
Tarsa Therapeutics completed Phase III of the ORACAL clinical trial on TBRIA for the treatment of postmenopausal osteoporosis in February 2011. It was a multinational, three-armed, double-blind, double-dummy study, which enrolled 565 postmenopausal women with osteoporosis in six countries. The mean age of the subjects was 66.5 years.
Patients were randomised to calcitonin (0.2mg/d) tablets and placebo nasal spray, synthetic salmon calcitonin (200lU/d) and placebo tablets, or placebo tablets and placebo nasal spray respectively in the ratio of 4:3:2 for 48 weeks. All patients were given calcium (1,000mg/d) and vitamin D (800 lU/d) supplements.
Results of the study demonstrated that in postmenopausal women with osteoporosis, TBRIA was superior compared to a placebo and other nasal calcitonin spray in increasing the bone mineral density.
Most adverse events in the trial were modest, and the adverse events were mainly confined to gastrointestinal and were similar to the TBRIA and placebo arms of the study.
Clinical trials on calcitonin-salmon for treatment of osteopenia
Tarsa Therapeutics also conducted a Phase II clinical trial on TBRIA for the treatment of osteopenia, which may be developed in the future.
The study evaluated the ability of the drug to improve bone mineral density (BMD) at the lumbar spine (LS) in women with osteopenia at increased risk of fracture. Study results showed that patients treated with TBRIA produced statistically significant improvements in BMD at the lumbar spine.
Marketing commentary
Based in Philadelphia, US, Tarsa Therapeutics develops novel therapies for the prevention and treatment of osteoporosis and associated bone diseases. The company’s current development pipeline also includes Ostora, an oral calcitonin tablet for the treatment of postmenopausal osteoporosis.