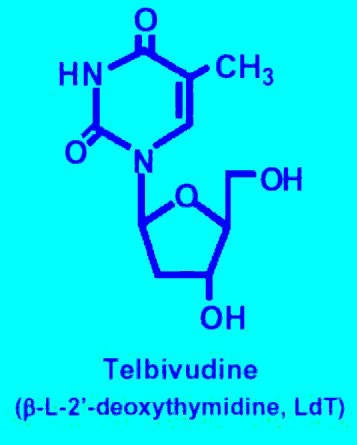
The product of a joint collaborative programme between Idenix Pharmaceuticals and Novartis Pharma, telbivudine (oral L-deoxythymidine) is a new once-daily treatment for chronic Hepatitis B Virus (HBV) infection. A highly specific and selective inhibitor of HBV replication in vitro, telbivudine specifically targets HBV DNA polymerase without inhibiting human cellular polymerases. In contrast to other nucleoside antiviral agents, telbivudine is an HBV-specific nucleoside analogue.
In October 2006, Idenix announced the approval of telbivudine by the US Food and Drug Administration. The company had filed a New Drug Application for the drug in January 2006. The drug is being sold in the US under the brand name Tyzeka. Outside the US, the drug is being sold under the brand name Sebivo.
In September 2006, telbivudine was approved in Switzerland. Novartis obtained regulatory approvals for the drug in several countries including Canada, Australia, South Korea, Latin America and several countries in Asia.
In April 2006, the European Commission approved telbivudine, which enabled the drug to be sold in 27 countries of the EU. Idenix had submitted a Marketing Authorization Application to the European Medicines Agency in March 2006.
In March 2007, telbivudine obtained regulatory approval from the Chinese State Food and Drug Administration. Idenix carried out additional Phase III clinical trials in China involving 332 patients to support the approval.
The burden of chronic HBV infection
HBV, sometimes termed serum hepatitis, is one of several viruses that can cause infectious hepatitis, a disease of the liver. Among the most common infectious diseases in the world, HBV is transmitted through contact with infected blood and other body fluids as well as from mother to child. Chronic HBV infection develops when the host’s immune response fails to eradicate the primary infection.
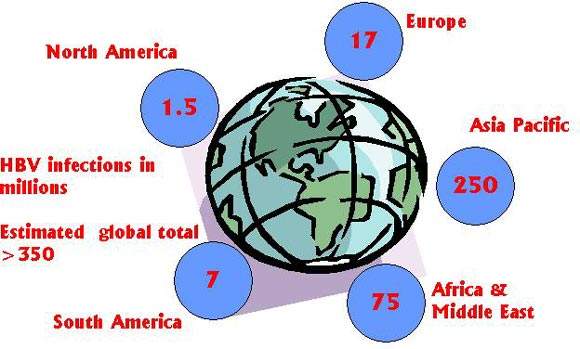
Estimates suggest that worldwide 350 million people have chronic HBV infection, placing them at increased risk of liver cirrhosis (scarring), hepatocellular carcinoma (liver cancer), liver failure and death. Prevalence is particularly high in Asia: China alone accounts for about one-third of all cases of chronic HBV infection.
While active immunisation against HBV infection is effective in preventing a primary infection, it is of no effect once HBV infection becomes established. Currently, over a million deaths occur annually from HBV-related chronic liver disease.
Meeting the challenge of drug resistance
In patients with chronic HBV infection, high levels of viral replication increase the risk of disease progression. Treatment is therefore designed to achieve sustained suppression of HBV replication.
Current treatments for chronic HBV infection include injectable interferon-alpha and the oral nucleotide analogues lamivudine, adefovir and entecavir. Interferon-alpha has been available for over ten years and provides sustained viral suppression and improvements in liver disease. However, it is not effective in all HBV patients and can cause potentially serious side effects.
More recently, treatment has expanded to include nucleotide analogues that directly inhibit HBV replication. These drugs can be taken orally, are effective in a high proportion of patients, and are generally well tolerated. However, drug resistance is a problem with these agents leading to viral breakthrough and increased risk of liver disease. Of the available drugs, lamivudine has the least favourable resistance profile; up to 70% of patients develop drug resistance within three to four years of treatment.
New, cost-effective oral treatments with increased antiviral potency, good tolerability, and a reduced propensity to induce resistance are clearly needed to improve the long-term prognosis of the many patients with chronic HBV infection.
Telbivudine shows efficacy in Phase III GLOBE registration trial
The efficacy and safety of telbivudine have been investigated in a series of clinical trials, culminating in the international Phase III GLOBE registration trial. Reputedly the largest HBV registration trial, GLOBE enrolled over 1,350 patients with chronic HBV infection.
Designed to compare the efficacy and safety of telbivudine 600mg with lamivudine 100mg in patients with HBe-Ag-positive and HBe-Ag-negative compensated chronic HBV over a two-year period, the trial showed that treatment with telbivudine was at least as effective as lamivudine.
Therapeutic response at one year (primary endpoint) was assessed in relation to a combination of viral suppression (serum HBV DNA suppression <100,000 copies/ml) and either improved liver function (ALT normalisation) or loss of detectable HBeAg.
Results from the GLOBE trial appear consistent with earlier clinical studies and suggest that treatment with telbivudine is not only effective but also safe and well tolerated.
The efficacy and safety of telbivudine is being assessed in an additional Phase III trial in HBe-Ag-positive and HBe-Ag-negative decompensated chronic HB. Again it is being compared with lamivudine. Results of the study are expected in April 2010.
Marketing commentary
The advent of oral nucleotide analogues represents an important advance in the treatment of chronic HBV infection, a disease that affects about 5% of the world’s population. Significant opportunities exist in the HBV market for more drugs of this and other classes that can offer greater viral suppression, are well tolerated, and slower to induce resistance.
Longer-term, the market for HBV therapies is expected to evolve towards the use of combination therapies, analogous to the use of HAART in HIV infection.