Under development by NeuroSearch, a Danish pharmaceutical company, tesofensine is a novel treatment for obesity. A serotonin-noradrenaline-dopamine reuptake inhibitor, tesofensine was originally in development for the treatment of neurological disorders such as Parkinson’s disease (PD) and Alzheimer’s disease.
Although tesofensine failed to demonstrate efficacy in PD trials, trial participants who were overweight achieved significant weight loss. This led the company to switch development of tesofensine to obesity.
Tesofensine is in Phase III trials evaluating it as an anti-obesity medication. The protocol of the first Phase III trial was approved by the US Food and Drug Administration in the first half of 2010.
Obesity-related disorders
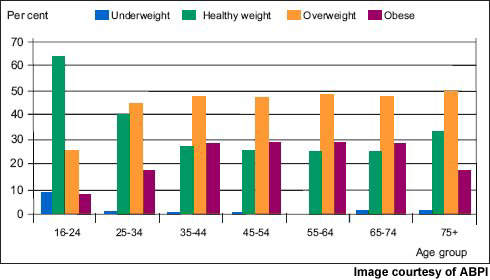
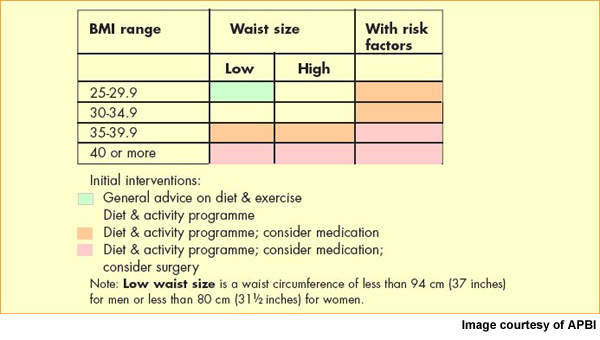
Now the most common nutritional disorder in western industrialised countries, obesity is defined as a body mass index (BMI) of greater than 30. Data from the World Health Organisation’s MONICA project show that in some parts of Europe over 70% of men aged 55–64 years are clinically obese or overweight (BMI >25) and almost 70% of women in this age group.
One in five of all Americans is obese and one in three overweight. Furthermore, increasing rates of childhood obesity are likely to exacerbate the trend towards increasing obesity in adulthood.
There is a strong association between obesity and increased risk of cardiovascular disease and diabetes and possibly certain cancers, such as breast and colorectal cancer.
The dramatic rise in the incidence of type 2 diabetes is due largely to the increased prevalence of obesity. Increases in body weight lead to changes in blood lipid and cholesterol levels, predisposing to increased risk of atherosclerosis.
Prevalence of obesity in the US and Europe has reached epidemic levels and, not surprisingly, has stimulated the search for new weight loss drugs.
Therapeutic targets for obesity
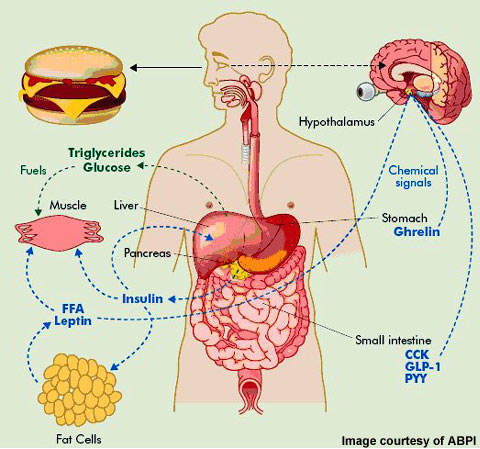
In the development of anti-obesity medication various therapeutic targets have been identified. They include serotonin and noradrenaline reuptake inhibitors (so-called anorectic agents), lipase inhibitors, b3-adrenoreceptor agonists, leptin agonists and melanocortin-3 agonists among others.
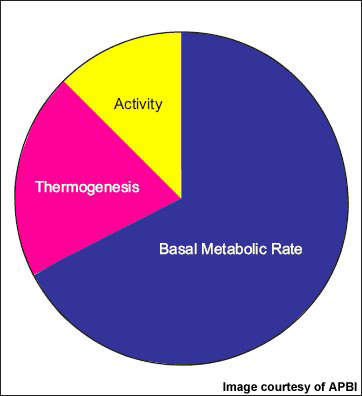
NeuroSearch’s tesofensine, an inhibitor of pre-synaptic uptake of the neurotransmitters serotonin, noradrenaline and dopamine, acts primarily as an appetite suppressant with concomitant effects on fat oxidation and resting energy expenditure. Clinical trial data suggests it may have the potential to achieve greater reductions in weight to that seen with currently approved weight loss agents.
Efficacy demonstrated in early trials
Tesofensine has demonstrated evidence for efficacy in several Phase II trials. In the 203-patient TIPO-1 study, participants were prescribed an energy-restricted diet before randomisation to treatment with tesofensine 0.25mg (n=52), 0.5mg (n=50) or 1.0mg (n=49), or placebo (n=52) once daily for 24 weeks.
At the end of the 24-week treatment period, tesofensine 0.25mg, 0.5mg and 1.0mg and diet induced a mean weight loss of 4.5%, 9.2% and 10.6% respectively. This compared with a mean weight loss of 2.0% for diet and placebo, a statistically significant difference (p<0·0001).
In the TIPO-4 trial, a 48-week open-label extension to the TIPO-1 trial, preliminary results suggest that weight loss with tesofensine is sustained. After an initial eight-week washout period, patients continuing with 0.5mg tesofensine achieved a total mean weight loss of 13–14kg at 24 weeks.
Additionally, previous placebo recipients switched to tesofensine 0.5mg lost approximately 9kg over the same period.
Dose-dependent adverse gastrointestinal effects were observed with tesofensine in the clinical trials in addition to increases in blood pressure and heart. However, at the anticipated therapeutic dose of 0.5mg, discontinuations for adverse effects with tesofensine were similar to placebo (8%).
In TIPO-2, 32 obese patients with their BMI values ranging from 28 to 35 were enrolled and treated for a period of 14 days. Patients were administered either Tesofensine or a placebo. The drug reduced the desire to eat and the satiety levels were found to be increased.
The Phase III trials will include four placebo-controlled studies and will enroll between 5,000 to 7,000 patients including those having type 2 diabetes and hypertension. Two of the four trials will be conducted for the obesity studies each for a period of one year. The trials will also include a two-year study to observe the safety and efficacy of the drug on the cardiovascular system. The trials began in 2011.
Marketing commentary
The market for weight-reducing drugs has had a somewhat chequered history, characterised by major product withdrawals because of safety concerns. Indeed, side effects have been a major concern with all currently available anti-obesity medications, as epitomised by the recent withdrawal of Acomplia (rimonabant) from the European market.
If tesofensine succeeds in phase III trials and is filed for regulatory approval, attention is likely to focus on its safety and tolerability profile. Regulatory authorities have been particularly concerned about the incidence of psychiatric adverse effects with recently developed anti-obesity medications.