Tremfya™ (guselkumab) is a biologic approved for the treatment moderate-to-severe plaque psoriasis in adults.
The human monoclonal antibody (mAb) was developed by US-based company Janssen Biotech, which submitted a biologics license application (BLA) to the US Food and Drug Administration (FDA) in November 2016.
The drug was developed using the in-licensed MorphoSys’ HuCAL antibody library technology.
Janssen received the FDA’s approval in July 2017 after an expedited regulatory review following the application of a priority review voucher. The European Commission (EC) approved Tremfya in November 2017.
The drug was approved by South Korea’s Ministry of Food and Drug Safety (MFDS) in April 2018 for the treatment of moderate-to-severe adult plaque psoriasis.
Tremfya was also approved for the treatment of moderate-to-severe forms of psoriasis and psoriatic arthritis in Japan in the same month. Taiho Pharmaceutical holds the distribution rights to the drug in Japan. It was later approved for the treatment of patients with palmoplantar pustulosis in Japan in November 2018.
The drug was also approved in Australia, Brazil and Canada.
Plaque psoriasis disease details
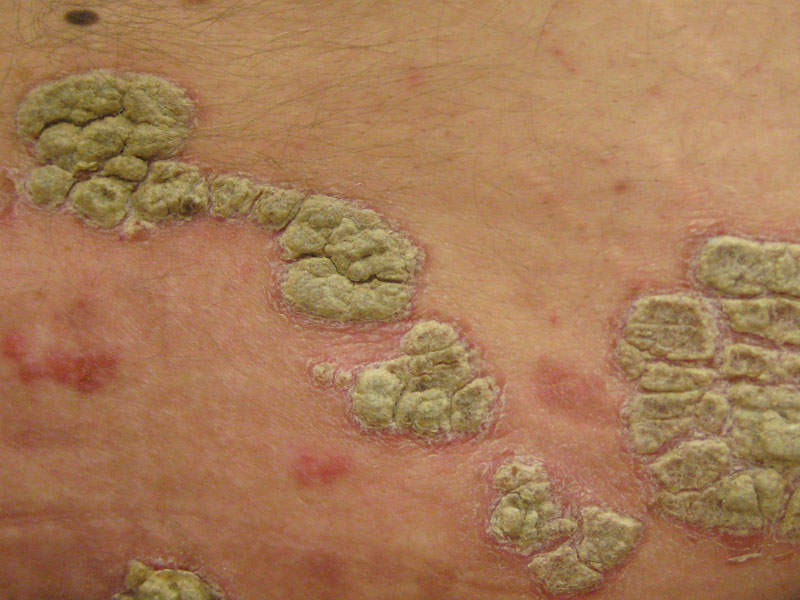
Plaque psoriasis is an autoimmune inflammatory disorder characterised by inflamed red lesions or plaques that are itchy and painful. The plaques are commonly found on the scalp, knees, elbows and lower back.
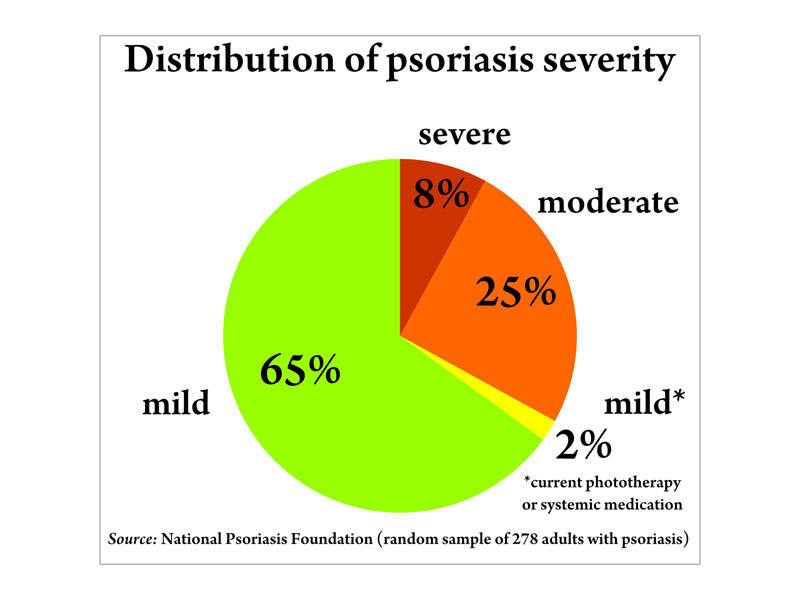
An estimated 7.5 million people in the US are affected by plaque psoriasis, with 80% of patients suffering from a mild-to-moderate form of the disease. Around 20% have a moderate-to-severe form.
Tremfya’s mechanism of action
Tremfya contains a mAb that selectively inhibits the release of pro-inflammatory cytokines and chemokines called interleukin (IL)-23.
The drug is available in 100mg / ml injections that can be administered subcutaneously.
Clinical trials on Tremfya
The FDA’s approval of Tremfya was based on results obtained from a clinical development programme, which included more than 2,000 patients in the Phase III VOYAGE 1, VOYAGE 2, and NAVIGATE studies.
The VOYAGE 1 clinical trial was conducted across 101 sites, while the VOYAGE 2 trial was conducted in 115 sites, including Canada and the US.
Results from the two studies showed that patients with moderate-to-severe plaque psoriasis treated with Tremfya showed significant efficacy. Patients treated with Tremfya also achieved 90% clearer skin, while more than 80% of patients demonstrated either clear or almost cleared skin at week 16.
The clinical studies showed improvement in Tremfya-administered patients with psoriasis on the scalp and a decrease of symptoms such as itching, pain, stinging and burning at week 16.
The clinical studies further demonstrated that nine out of ten patients treated with Tremfya achieved clear skin and achieved a Psoriasis Area and Severity Index (PASI) score of 90 at week 28, which was continued to week 48.
In the VOYAGER 2 clinical study, Tremfya was compared with Humira®. The study results showed more than seven out of ten patients treated with Tremfya reported at least 90% clear skin compared to four out of ten patients treated with Humira. The patients treated with Tremfya achieved superior skin clearance (PASI 90) compared to Humira.
The NAVIGATE clinical study was a randomised, double-blind, multi-centre study that evaluated the efficacy and safety of Tremfya compared to Stelara® (ustekinumab) in patients that continued to experience mild-to-severe skin symptoms following 16 weeks of treatment with Stelara.
Patients that switched to Tremfya showed greater improvement in their psoriasis between weeks 28 and 40. Approximately 31% of the patients treated with Tremfya were considered cleared or almost cleared compared to 14% of Stelara-treated patients.