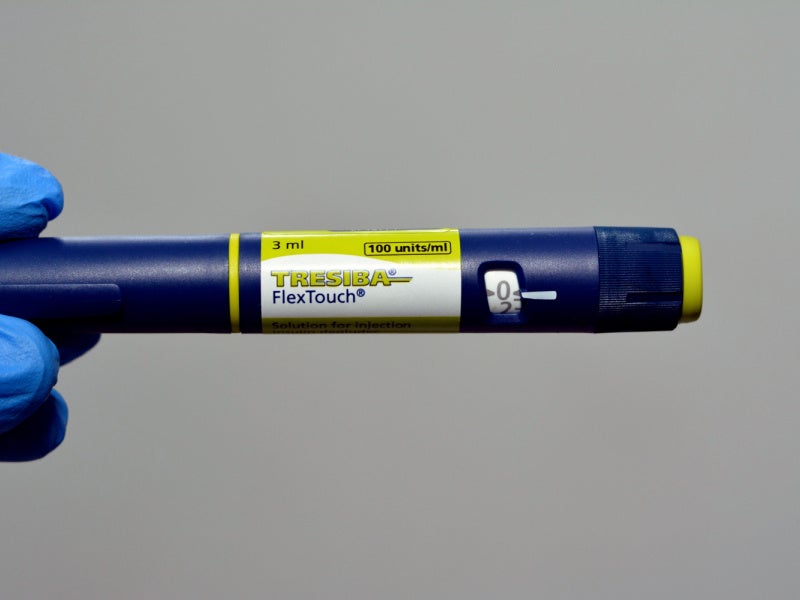
Tresiba® (insulin degludec) is the first long-acting human insulin indicated for the treatment of Type 1 and Type 2 diabetes in patients aged one year or older. It is also the first basal insulin that allows patients to dose at any time of the day.
Discovered and developed by Novo Nordisk, Tresiba® can be used alone or in combination with oral anti-diabetic medicines or bolus insulin and acts for more than 42 hours.
Studies conducted on Tresiba® demonstrated that even at lower doses, the drug achieved reduced long-term blood glucose levels (HbA1c) and greater fasting plasma glucose reduction.
Tresiba® is available in the form of a 10ml vial for injection with 100 units/mL. It is also available as the FlexTouch device, a prefilled insulin pen containing two concentrations of 100 units/mL and 200 units/mL.
Regulatory approvals on Tresiba
Tresiba® was first approved in Japan in September 2012. The European Medicines Agency (EMA) approved the drug in 2013.
The new drug application of Tresiba® as a once-daily, long-acting basal insulin was approved by the US Food and Drug Administration (FDA) in September 2015, making it the first basal insulin to be approved by the FDA in ten years.
The drug was launched in the US in January 2016. In December 2016, the FDA approved an extended indication of Tresiba® for use in diabetic patients as young as one year old.
In September 2017, the European Union approved a label update to reflect the drug’s effect in significantly reducing the risk of severe hypoglycaemia based on the DEVOTE trial. The label update was approved by the FDA in March 2018.
Tresiba® was approved by Health Canada in August 2017. In November 2022, Health Canada approved a label update for the drug for use in pregnant women with diabetes.
In July 2022, Novo Nordisk announced the approval of the unbranded biologic version of insulin degludec by the FDA.
Diabetes mellitus and insulin degludec
Diabetes mellitus is a condition in which the body either doesn’t produce enough insulin or is unable to use it properly. As insulin plays a key role in controlling sugar levels in the blood, irregular levels lead to an excess buildup of sugar. High blood sugar leads to serious health problems.
Chronic diabetes includes Type 1 and Type 2 diabetes, of which Type 2 is the most common and accounts for approximately 90% to 95% of all diabetes cases. Diabetes is a serious health problem, affecting more than 37 million people in the US alone.
Tresiba’s mechanism of action
Tresiba® contains an active ingredient called insulin degludec, which, similar to any other insulin, regulates glucose metabolism. It reduces blood glucose levels by stimulating peripheral glucose uptake and controlling hepatic glucose production.
When injected, insulin degludec forms stable depot of multi-hexamers in subcutaneous tissue. From there, it is slowly absorbed into the systemic circulation, resulting in a prolonged time-action profile.
Clinical trials on Tresiba
The FDA approval for Tresiba® was based on the results from a clinical trial programme known as Begin, which included nine randomised, controlled, treat-to-target, open-label trials conducted on Type 1 and Type 2 diabetics from more than 40 countries.
The first three studies, A, B and C, were conducted on patients with Type 1 diabetes. Subjects in these randomised, open-label, treat-to-target, active-controlled trials were treated once daily with Tresiba® in combination with mealtime insulin.
The next six studies, D to I, also randomised, open-label, treat-to-target, active-controlled trials, were conducted on Type 2 diabetic patients. The subjects were administered once-daily Tresiba® in combination with mealtime insulin or a common oral anti-diabetic.
Results of all nine studies demonstrated that patients treated with Tresiba® achieved levels of glycemic control similar to those achieved through other insulin medications such as Lantus (insulin glargine) and Levemir (insulin detemir) and also achieved statistically significant improvements compared to sitagliptin.
The label extension approved by the FDA and Health Canada for severe hypoglycaemia was based on the DEVOTE trial, which was conducted in more than 7,000 patients with Type 2 diabetes. The trial showed that treatment with Tresiba® reduced the risk of severe hypoglycaemia by 40% compared to insulin glargine.
Health Canada’s label update for use in pregnant women with diabetes was based on the results of the EXPECT Pregnancy Trial, which compared Tresiba® with Levemir® (insulin detemir), both in combination with NovoRapid® (insulin aspart) in 255 women with Type 1 diabetes. The study found that Tresiba® was non-inferior to Levemir® in terms of A1C control in pregnant women with Type 1 diabetes.
Marketing commentary on Novo Nordisk
Founded in 1923 and headquartered in Denmark, Novo Nordisk has been innovating and leading in diabetes care for more than 90 years. It employs more than 59,000 people in 80 countries and markets its products in more than 170 countries. The company introduced pen needles for injectable diabetes therapies approximately 30 years ago.