Tyrvaya™ (varenicline solution) is a multi-dose nasal spray indicated for the treatment of signs and symptoms of dry eye disease. The novel formulation treats dry eye disease without being administered on the already irritated surface of the eyes.
Developed by US-based clinical-stage biopharmaceutical company Oyster Point Pharma, Tyrvaya is available as a nasal spray delivering 0.03mg (0.05ml) of varenicline in each spray. This is recommended to be administered once in each nostril twice a day, 12 hours apart.
Regulatory approvals for Tyrvaya
In December 2020, Oyster Point submitted a 505(b)(2) new drug application (NDA) for Tyrvaya (OC-01, varenicline) nasal spray to treat the signs and symptoms of dry eye disease to the US Food and Drug Administration (FDA).
The NDA was accepted in March 2021, while the FDA approved Tyrvaya nasal spray 0.03mg for the indication in October 2021.
Dry eye disease causes and symptoms
Dry eye disease is a multifactorial, chronic, progressive ocular surface condition characterised by the disruption of tear film homeostasis.
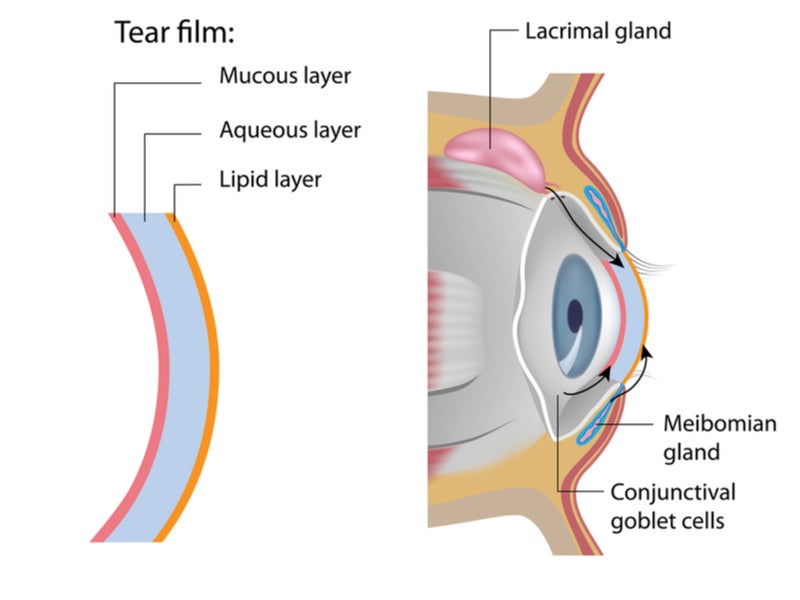
The disease affects around 340 million individuals worldwide and is becoming extremely prevalent. A healthy tear film has three layers comprising fatty oils, aqueous fluid and mucus, which maintain clear vision by keeping the eye surface protected, lubricated and smooth.
Tear film comprises growth factors and antibacterial components and washes away foreign particles to minimise infection risk.
Symptoms of dry eye disease include persistent stinging, scratchy, burning sensation, pressure in the eyes, light sensitivity, blurred vision, pain, and eye tiredness.
Tyrvaya’s mechanism of action
Tyrvaya is a highly selective cholinergic agonist that binds to cholinergic receptors with high affinity. It activates the trigeminal parasympathetic pathway, accessible within the nose, resulting in enhanced basal or natural tear film production.
The trigeminal parasympathetic pathway innervates lacrimal glands, meibomian glands and goblet cells, which produce basal tear film and keep the tear film in a state of homeostasis. The drug’s exact mechanism of action is still unknown.
Clinical trials on Tyrvaya
The FDA’s approval of Tyrvaya nasal spray was supported by the ONSET-1, ONSET-2 and MYSTIC clinical trials, which enrolled more than 1,000 adult patients with symptoms of mild, moderate or severe dry eye disease.
Phase IIb ONSET-1 and Phase III ONSET-2 were both multi-centre, randomised, double-blind, vehicle-controlled studies conducted to study the drug’s efficacy.
In the ONSET-1 study, 182 patients were randomised to receive either 0.006mg varenicline solution or 0.03mg Tyrvaya, or 0.06mg varenicline solution or vehicle, one spray in each nostril twice a day.
The ONSET-2 study randomly assigned 758 patients to receive either 0.03mg Tyrvaya, 0.06mg varenicline solution, or vehicle, one spray in each nostril twice a day.
Both trials’ primary measure outcome was basal tear production. This was evaluated by the change in anaesthetic Schirmer’s score from baseline at week four, using a Schirmer’s strip (0-35 mm).
The ONSET-1 and ONSET-2 trials’ secondary endpoint was eye dryness, which was assessed by the change in the eye dryness score (EDS) from baseline. EDS was evaluated in the controlled adverse environment (CAE®) and the clinic environment.
At week four, 52% of patients treated with Tyrvaya in ONSET-1 and 47% of patients in the ONSET-2, improved their Schirmer’s score by equal to or more than 10mm from baseline, compared to 14% and 28% of vehicle-treated patients respectively.
The mean change in Schirmer’s score for patients treated with Tyrvaya was 11.7mm and 11.3mm, compared to 3.2mm and 6.3mm in the vehicle-treated patients in the ONSET-1 and ONSET-2 trials respectively, at week four.
In the CAE, the observed mean EDS change from baseline at week three in Tyrvaya-treated patients was -16mm and -10.3mm, compared to -4.4mm and -7.4mm in vehicle-treated patients in ONSET-1 and ONSET-2 respectively.
In the clinic environment, the mean change from baseline in EDS at week four in Tyrvaya-treated patients was -18.9mm and -19.8mm, compared to -5.4mm and -15.4mm in vehicle-treated patients in ONSET-1 and ONSET-2 respectively.
The most frequent adverse reactions observed in patients during the clinical trials were sneezing, cough, throat irritation and instillation-site (nose) irritation.