Viibryd (Vilazodone) is an antidepressant manufactured for the treatment of adults with major depressive disorder (MDD). The drug was developed by Trovis Pharmaceuticals (formerly known as PGxHealth), a wholly owned subsidiary of Clinical Data. It was approved by the US FDA on 21 January 2011.
In February 2011, Forest Laboratories and Clinical Data entered into a merger agreement under which Forest Laboratories will acquire Clinical Data. The acquisition was completed in April 2011.
Forest Laboratories launched Viibryd in the US market in June 2011.
Major depressive disorder
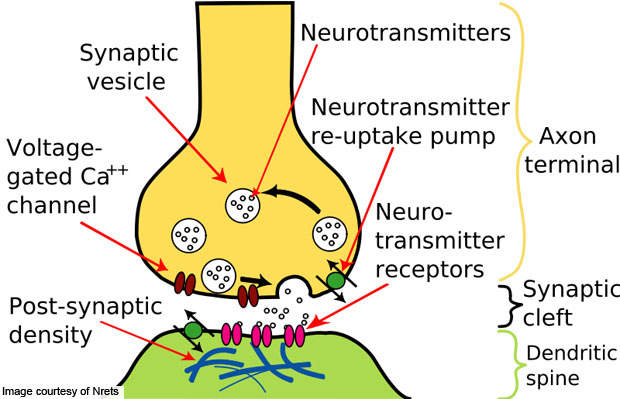
MDD is mental disorder accompanied by low mood and self-esteem. The condition is caused by the imbalance of endogenous chemicals in the brain commonly known as neurotransmitters, which transmit signals from a neuron to a target cell across the junction between two neurons (called synapse).
The World Health Organization (WHO) estimates that MDD affects more than 18 million people in the US alone. It is estimated that approximately 3.4% of MDD patients in the US commit suicide. MDD accounts for over 60% of suicide cases in the US.
Viibryd inhibits serotonin reuptake
Viibryd is believed to work by inhibiting the reuptake of serotonin, a neurotransmitter in the central nervous system. The mechanism of the drug is not completely understood.
In the brain, messages pass from one nerve cell to another. The sender is called the pre-synaptic cell and the recipient is known as the postsynaptic. The junction between the pre-synaptic and postsynaptic cells is referred to as the chemical synapse.
The signal from the neurotransmitters is relayed to the postsynaptics via receptors. While passing through the pre-synaptic to postsynaptic cells, about 10% of the neurotransmitters are lost during passage. The remaining reuptake into the pre-synaptic cell again. By inhibiting the reuptake of serotonin, stimulation on the receptors can be increased.
Viibryd stimulates the postsynaptic cells by inhibiting the reuptake of serotonin. Due to this, serotonin remains in the synaptic gap for a longer period, which in effect stimulates the receptors of the postsynaptic cell repeatedly.
The drug is also a partial agonist of serotonergic 5-HT1A receptors.
Clinical trials
Clinical trials conducted on Viibryd include two eight week and one 52 week open label phase III clinical trials on a total of 2,177 MDD patients.
The two eight week randomised, placebo-controlled and double blind phase III clinical trials were conducted from February 2006 to March 2009 across multiple centres in the US. The studies recruited 861 MDD patients who met DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders) criteria and aged between 18 to 70 years. The patients were treated with a 40mg dose of Viibryd once daily.
The primary outcome measure was to find the change in MADRS (Montgomery-Asberg Depression Rating Scale) from baseline to eighth week. MADRS is a proven efficient and practical measure of depression. The secondary outcome measures were to find the change in HAM-D 17 and 21 (Hamilton Rating Scale for Depression) and CGI-I (Clinician’s Global Impression of Improvement) levels from the baseline to eight week period. HAM-D is designed to assess the depressive symptom severity in patients with depressive disorder.
The first set of eight week phase III trials was conducted between February 2006 and May 2007 on 410 MDD patients. The second phase III trials were conducted from March 2008 to March 2009 with 481 MDD patients.
Both the trails proved the efficacy and safety of Viibryd in treating MDD patients in comparison with a placebo. The most common adverse effects in Viibryd-treated patients were nausea, diarrhoea, insomnia and vomiting.
The 52-week open label clinical study was conducted between December 2007 and May 2009. It enrolled 599 MDD patients in 38 centres across US. The primary outcome measure was the assessment of adverse effects, Columbia-Suicide Severity Rating Scale (C-SSRS), ECGs, laboratory parameters and vital signs.
The secondary outcome measure included finding the effectiveness of Viibryd using CGI severity illness, MADRS and CGI improvement scales.
The patients were administered with 10mg daily dose of Viibryd for the first two weeks and 40mg a day thereafter. The results proved that Viibryd is safe and tolerable.