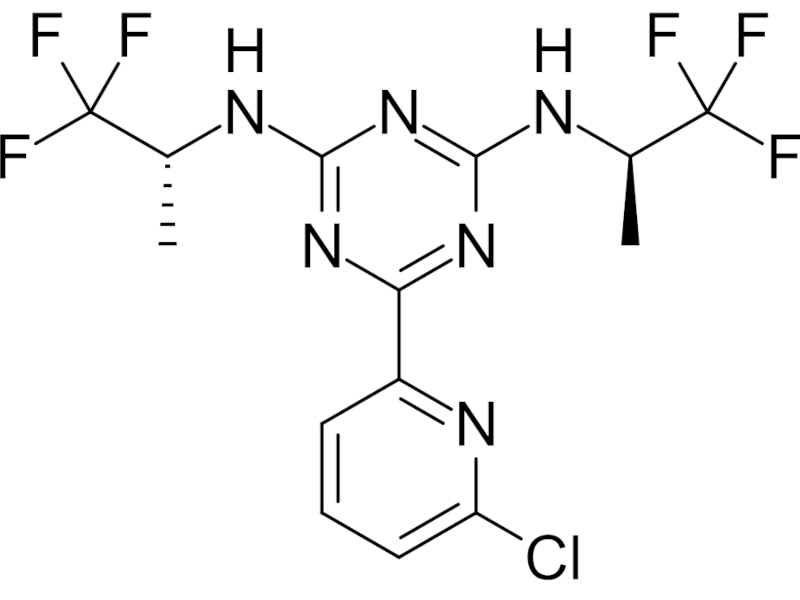
VORANIGO® (vorasidenib) is an isocitrate dehydrogenase (IDH) inhibitor indicated for the treatment of adults and children aged 12 years and older with specific brain tumours such as astrocytoma or oligodendroglioma with susceptible IDH1 or IDH2 mutations, after surgical intervention.
Vorasidenib was originally part of the pipeline of Agios Pharmaceuticals, a pharmaceutical company based in the US.
In April 2021, Servier Pharmaceuticals, a US subsidiary of the French pharmaceutical company Servier Laboratories, acquired Agios’ oncology portfolio, thereby adding vorasidenib and several other drugs to its development pipeline.
VORANIGO® marks Servier’s sixth approved drug in the arena of IDH-mutant targeted treatments.
VORANIGO® is available as white to off-white, film-coated tablets for oral administration, with dosage strengths of 10mg (round) and 40mg (oblong).
Regulatory approvals for VORANIGO
The US Food and Drug Administration (FDA) granted approval to VORANIGO® in August 2024, following the acceptance of the new drug application (NDA) for VORANIGO® with priority review in February 2024.
The European Medicines Agency granted accelerated assessment for the marketing authorisation application (MAA) of VORANIGO® in February 2024.
The European Commission’s approval for VORANIGO® is expected in the latter half of 2024.
Glioma causes and symptoms
Gliomas are tumours originating from glial or precursor cells within the central nervous system (CNS).
Diffuse gliomas with IDH mutations are the most prevalent primary brain tumours in adults aged under 50. These tumours are incurable with existing treatments and, if left untreated, they will proliferate and invade healthy brain tissue.
Adult-type diffuse gliomas are categorised into three types: astrocytoma, oligodendroglioma and glioblastoma, according to the 2021 World Health Organization’s classification of CNS tumours and the National Comprehensive Cancer Network treatment guidelines.
Astrocytoma develops from the star-shaped cells called astrocytes in the cerebrum and account for 60% of brain tumours.
Oligodendrogliomas, a variety of diffusely infiltrating glioma, make up around 5% of primary intracranial tumours. These tumours frequently affect the cortical grey matter and are often found in the frontal lobes. Astrocytoma and oligodendroglioma not only impact the brain but can also affect the spinal cord.
Gliomas can disrupt normal brain function, leading to a range of symptoms including alterations in mental function, seizures, speech difficulties, nausea and numbness or weakness in parts of the body.
VORANIGO’s mechanism of action
Vorasidenib acts as a small molecule, selective, highly brain-penetrant dual inhibitor, targeting mutant IDH1 and IDH2 enzymes.
It effectively inhibits both the wild-type and mutant variants of IDH1, including R132H, as well as the wild-type and mutant variants of IDH2 in vitro.
Furthermore, in cell-based assays and in vivo tumour models that express mutated IDH1 or IDH2 proteins, vorasidenib has reduced the production of 2-hydroxyglutarate (2-HG) and partially reestablished cellular differentiation.
Clinical trials on VORANIGO
The FDA’s approval of VORANIGO® was based on the pivotal outcomes of the global, randomised, multi-centre, double-blind, placebo-controlled Phase III clinical trial, INDIGO.
It investigated the efficacy of VORANIGO® compared to a placebo in patients with residual or recurrent Grade 2 glioma with an IDH mutation, who have undergone surgical treatment as their sole therapy.
A total of 331 patients were allocated randomly in a 1:1 ratio for the administration of either 40mg VORANIGO® or a placebo orally once daily.
The primary endpoint of the study was progression-free survival (PFS). The median PFS times in patients treated with VORANIGO® and placebo were 27.7 months and 11.1 months respectively.
Additionally, VORANIGO® demonstrated a reduction in tumour volume by an average of 2.5% every six months, whereas the tumour volume in patients assigned to the placebo arm increased by an average of 13.9% over the same period.
The drug demonstrated efficacy across various mutations, leading to tumour shrinkage in IDH1/2-mutant diffuse glioma.
The most common adverse events reported among the patients were fatigue, headache, COVID-19, musculoskeletal pain, diarrhoea, nausea and seizures.
Additional data from the INDIGO study shows that vorasidenib helps preserve quality of life, maintain stable neurocognitive function, and control seizures in patients with IDH-mutant diffuse gliomas.