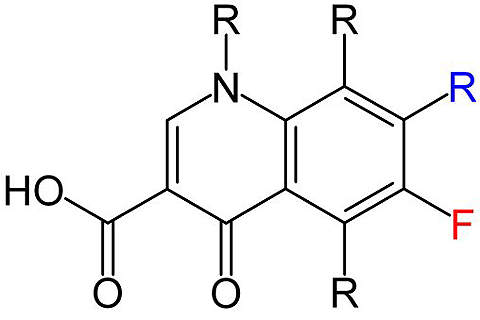
Voreloxin is a quinolone derivative being investigated for the treatment of various cancers. The drug is being developed by California-based Sunesis Pharmaceuticals.
In November 2009, the US Food and Drug Administration (FDA) gave an orphan drug designation to voreloxin for the treatment of acute myeloid leukaemia (AML). Sunesis announced positive results from Phase II clinical trials for AML and ovarian cancer in June 2010; the company plans to launch Phase III trials for AML in the second half of the year.
Acute myeloid leukaemia and ovarian cancer
AML is a rapidly progressing and fatal type of cancer. In 2009, 13,000 new cases were recorded in the US alone. The disease is characterised by an abnormal build up of immature blast cells in the bone marrow. Blast cells are white blood cells in the early stage of growth – in AML they do not develop completely. These abnormal cells rapidly increase in numbers, preventing the growth of red blood cells, white blood cells and platelets required by the body.
The disease normally affects adults over the age of 67. The most common symptoms of AML are fatigue, infections and excessive bleeding. If left untreated, AML can cause death within one year. The acute nature of the disease has given rise to an urgent need for new treatment methodologies. Current treatment options include chemotherapy, radiation therapy and surgery.
Ovarian cancer is the fifth biggest cause of cancer deaths in women in the US. In 2009, 21,550 new cases and 14,000 deaths due to ovarian cancer were reported in the county. The disease occurs when a cancerous tumour forms in the tissues of the ovary. The tumour can be one of two types: epithelial or germ cell.
Epithelial tumours develop in the epithelium, a tissue layer covering the ovaries, and account for 85-90% of all ovarian tumours. Germ cell tumours develop in the egg-producing cells of the ovary. This type of tumour normally occurs in younger women. The exact cause of ovarian cancer is not known but family history, breast cancer and DNA changes have been linked to the disease.
The recurrence rate for ovarian cancers is very high, and only 30% of patients survive for more than five years after contracting the disease. Symptoms include abdominal pressure, bloating, persistent nausea and lower back pain. Treatment options available for ovarian cancer include a combination of surgery and chemotherapy.
Voreloxin: a first-in-class anti-cancer quinolone derivative
Voreloxin has a dual anti-cancer action that combines DNA intercalation and the suppression of topoisomerase II activity. Topoisomerase II is a nuclear enzyme which is responsible for conformational (shape) changes in the DNA of cells. Voreloxin stops the cleaving and re-ligating of DNA caused by topoisomerase II in cancer cells. This damage leads to the G2 phase of cell division coming to a halt in cancer cells, followed by apoptosis (programmed cell death).
Compared to other topoisomerase II inhibitors, voreloxin has several unique properties. Since it is not a P-glycoprotein substrate, multidrug resistance is avoided. It is independent of the p53 gene family and has a chemically stable molecular structure. In addition, voreloxin has a better targeted approach to DNA damage. These properties make voreloxin ideal to be developed to treat a range of tumours.
Voreloxin’s clinical trials
Three Phase I clinical trials have been conducted for voreloxin. The first two studies, SPO-0001 and SPO-0002, involved patients with advanced solid cancers. The third study, SPO-0004, involved patients with advanced or refractory acute leukaemia. Results from the three studies showed that voreloxin was well tolerated; it demonstrated pharmacokinetic properties.
Three more studies were carried out: a Phase 1b/2 study tested voreloxin in combination with cytarabine in 69 patients with relapsed or refractory AML; a Phase II study tested voreloxin as a single therapy in 113 older adults with newly diagnosed and previously untreated AML; and a final Phase II study examined voreloxin’s effect on ovarian cancer in 143 women aged 18 years or older. The patients recruited for this third study included those who had already undergone platinum-based chemotherapy, but did not respond to the treatment, and patients who relapsed within six months of platinum-based therapy.
In June 2010, Sunesis announced positive clinical results from all of the Phase II studies. The patients in these studies demonstrated significant improvements in overall survival rates compared to standard treatment options. Sunesis announced that the positive results supported initiation of Phase III development of the drug.
The Phase III study of voreloxin will be a randomised, double-blind, placebo-controlled trial. It is expected to involve about 450 patients with first relapsed or primary refractory AML. The study is scheduled to begin in the second half of 2010, and will be conducted across various centres in the US and Europe.
Marketing commentary
Compared to current treatment methodologies available for AML and ovarian cancer, voreloxin uses a novel mechanism of action. The drug’s ability to cause cell death and stop DNA re-ligation promises avenues for the treatment of a range of cancers. In addition, voreloxin’s orphan drug designation means that if approved it will be eligible for seven years of market exclusivity in the US. It will also be exempted from user fees, which is expected to significantly boost Sunesis’s market position.