Vtama® (tapinarof) is a first-of-its-class, non-steroidal, topical medication indicated for the treatment of mild, moderate and severe plaque psoriasis in adults.
US-based biopharmaceutical company Dermavant Sciences developed the drug after acquiring all global rights to tapinarof, except in China, from GlaxoSmithKline (GSK) in July 2018. Under its agreement with GSK, Dermavant is responsible for the preclinical topical back-up and clinical development programmes for tapinarof.
In May 2021, Dermavant submitted a new drug application (NDA) to the US Food and Drug Administration (FDA) for the approval of Vtama. The FDA approved the drug in May 2022.
Vtama is available as topical, white to off-white cream, 1% to be applied to the affected areas once daily. One gram of Vtama cream contains 10mg of tapinarof.
Plaque psoriasis causes and symptoms
Plaque psoriasis is a chronic inflammatory skin disorder characterised by rashes, which appear as red or flaky white scales on the skin. Genetic and environmental factors are the prime causes of the disease.
Topical medications and light therapy are some of the treatments available to reduce the chronic inflammation caused by the disease.
More than 26 million people in the US and around 125 million people worldwide are estimated to be living with psoriasis. The disease affects individuals’ psychological health and quality of life.
Vtama’s mechanism of action
Tapinarof is a therapeutic aryl hydrocarbon receptor (AhR) modulating agent (TAMA). The exact mechanism by which the cream imparts therapeutic action on psoriasis is yet to be determined.
Clinical trials on Vtama
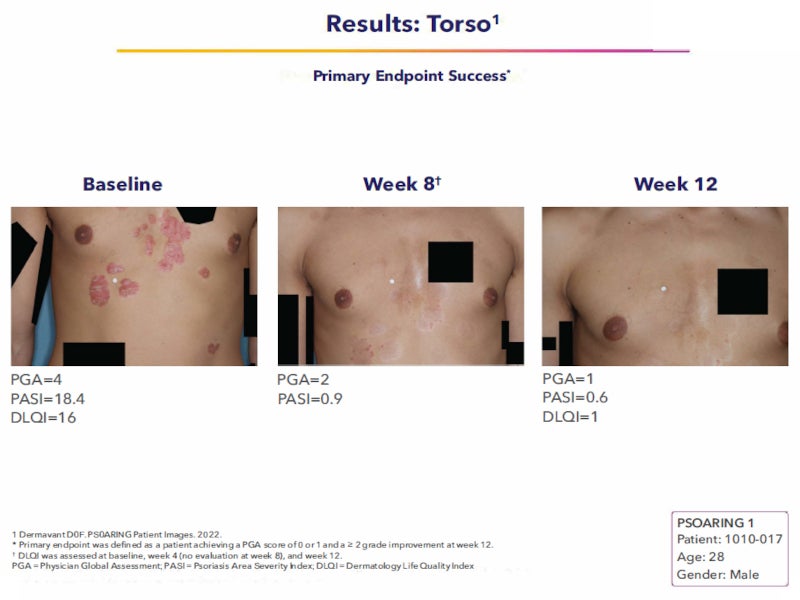
The FDA’s approval of Vtama was based on the data from two identical multi-centre, randomised, double-blind, vehicle-controlled, Phase III clinical trials named PSOARING-1 and PSOARING-2.
The trials were conducted to assess the safety and efficacy of Vtama cream for the treatment of adults with plaque psoriasis. A total of 1,025 individuals were enrolled in the trials and randomised in a 2:1 ratio to Vtama cream or vehicle cream for once-daily application for 12 weeks.
The primary endpoint of the studies was the patients’ clinical response after 12 weeks. Disease severity was categorised using the five-point Physician’s Global Assessment (PGA). Treatment success was defined as complete disease clearance (PGA=0) or almost clear skin (PGA=1) and at least a two-grade improvement from baseline.
In the PSOARING-1 study, 36% of patients treated with Vtama cream achieved clear or almost clear skin, compared with just 6% of patients treated with vehicle cream. In the PSOARING-2 study, 40% of the patients who were treated with Vtama cream achieved clear or almost clear skin versus 6% in vehicle cream.
The median duration of clear or almost clear skin after stopping treatment with Vtama cream was approximately four months. The intermittent use of Vtama cream demonstrated durability of response of up to 52 weeks.
Eligible patients who completed the PSOARING 1 or 2 trials were given a choice to participate in a Phase III long-term extension (LTE) study named PSOARING 3. Around 92% of eligible patients opted for the Phase III LTE study. The study conducted an additional open-label treatment with Vtama cream for 40 weeks, followed by a four-week follow-up.
In the LTE study, more than 40% of the participated patients achieved complete disease clearance (PGA=0) at least once during the study period. The patients received Vtama cream treatment for up to 52 weeks, following the completion of the LTE study. Around 81.7% of the patients considered Vtama cream to be more effective than previous topical treatments.
The safety and tolerability of Vtama cream were consistent through the PSOARING 1 and 2 and LTE studies.
The most common adverse reactions reported in patients treated with Vtama cream were folliculitis (red raised bumps around the hair pores), pain or swelling in the nose and throat, contact dermatitis, headache, itching, and influenza.
Vtama for atopic dermatitis (eczema)
Atopic dermatitis is a common inflammatory skin disease characterised by itchy, red, swollen and cracked skin, primarily affecting children. It affects up to 10% of adults and up to 30% of children worldwide. Dermavant has begun a pivotal Phase III clinical programme for tapinarof in atopic dermatitis (AD). The programme consists of two identical pivotal trials, ADORING 1 and ADORING 2, which will be followed by an open-label, LTE study named ADORING 3.
ADORING 1 and 2 are two identical, multi-centre, randomised, vehicle-controlled, double-blind, parallel-group studies to be conducted in North America.
Around 800 patients were enrolled across the two pivotal trials to evaluate the safety and efficacy of tapinarof cream for eight weeks compared with vehicle cream in patients aged two years and older with moderate to severe atopic dermatitis. The primary endpoint of both studies will be the patient treatment success defined as a Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD™) score of zero or one with at least a two-grade improvement from baseline at week eight.
The first patient in the trial was dosed in September 2021.
ADORING 3 is a long-term, open-label, extension study to evaluate the safety and efficacy of the drug in a 48-week study period, followed by a seven-day safety period.