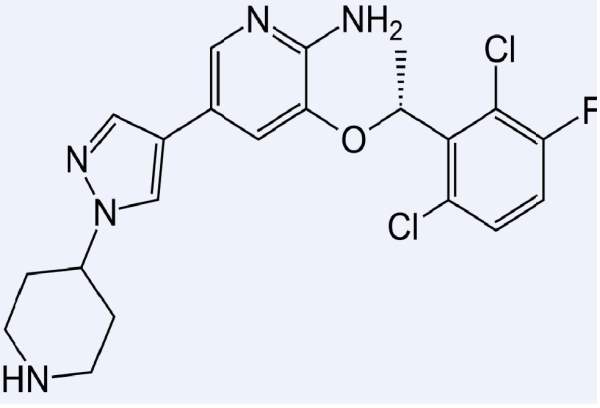
Xalkori (Crizotinib) is an oral drug indicated for the treatment of non-small cell lung cancer (NSCLC). The drug is developed and manufactured by Pfizer.
Crizotinib obtained orphan drug designation from the US FDA in September 2010. It was granted fast track designation in December 2010. Pfizer filed a new drug application in May 2011.
The FDA approved the drug for the treatment of metastatic anaplastic lymphoma kinase (ALK) positive NSCLC in August 2011. The approval was based on trial data of 255 patients enrolled in two clinical trials namely Phase II-Profile 1005 and Phase I-Study 1001.
Pfizer submitted a marketing authorisation application to the European Medicines Agency (EMA) in August 2011. The drug was reviewed by the EMA. In October 2012, Pfizer received conditional approval for Xalkori from the European Commission (EC) for the treatment of advanced NSCLC.
The drug also approved in Japan, Canada, Switzerland and South Korea. It is under regulatory review in several countries across the world.
Non-small cell lung cancer
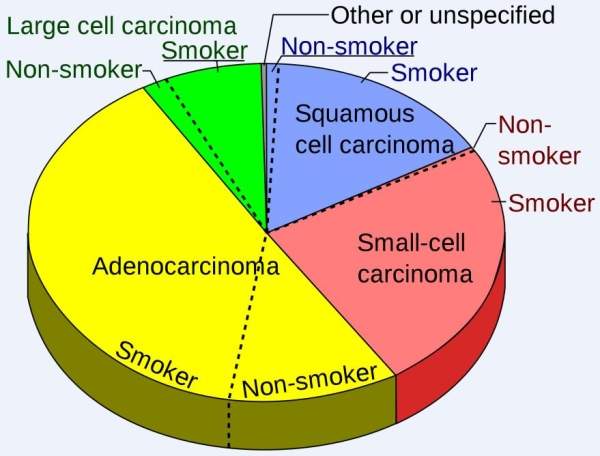
Non-small cell lung cancer (NSCLC) is the most common kind of lung cancer. The growth and progression of NSCLC is slower than that of small cell lung cancer. Adenocarcinomas, squamous cell carcinomas and large cell carcinomas are the different types of NSCLC.
NSCLC is usually caused by smoking. It also occurs in people who work near asbestos, products using chloride and formaldehyde, certain alloys, paints, pigments and preservatives.
Symptoms of NSCLC include coughing up blood, shortness of breath, wheezing, chest pain, loss of appetite, losing weight without trying and fatigue.
Around 45,000 patients worldwide are diagnosed with ALK positive NSCLC every year.
Crizotinib – mechanism of action
Crizotinib is an oral medication having the ability to inhibit ALK and c-MET.
ALK is an enzyme encoded by ALK gene. It can be carcinogenic due to mutation or due to formation of a fusion with another gene thereby leading to ALCL (anaplastic large-cell lymphomas), NSCLC or neuroblastoma.
After ALK inhibition, the cell signalling necessary for the growth of tumour and survival in various cell pathways is blocked.
Clinical trials
Study 1006, a Phase I study, is being conducted to evaluate safety, efficacy, pharmacokinetics and pharmacodynamics of Crizotinib in 70 NSCLC patients. The trial began in April 2010.
Around 175 patients will be enrolled in another Phase I trial being conducted to evaluate the safety of the drug and determine the maximum tolerable dose.
A Phase II study named PROFILE 1005 was initiated in 400 NSCLC patients in January 2010. The trial is being conducted to evaluate the safety and efficacy of the drug in ALK positive NSCLC patients. The trial is expected to be complete by September 2013.
Phase I/II study 1002 was initiated in January 2010 in 175 patients having NSCLC. It will evaluate the safety and efficacy of Erlotinib alone in comparison with Erlotinib in combination with Crizotinib. The enrolled patients are being administered either 150mg of erlotinib alone or 150mg of erlotinib in combination with 200-250mg of crazotinib. The primary end point is to determine the maximum tolerable dose and the progression free survival. The trial is expected to be complete by July 2014.
The FDA approval of Xalkori was based on the trial data of 255 patients from PROFILE 1005 and Study 1002.
The objective response rate (ORR) of PROFILE 1005 was 50%. The patients were treated for an average period of 22 weeks. Around 79% of tumour responses were observed during the first eight weeks.
In Study 1001, patients were treated for an average period of 32 weeks. The ORR was 61% and around 55% of this was observed during the first eight weeks.
Phase III development
Phase III trial PROFILE 1014 is currently being conducted in patients with NSCLC.
Around 334 NSCLC patients have been enrolled in the trial. The trial, which began in January 2011, will determine the anti-cancer effects of the drug and will compare them to those of the chemotherapy in ALK positive patients. Primary completion date of the study is October 2013. The entire trial is expected to be complete by December 2013.
The EC approval for Xalkori was based on the results of PROFILE 1007 which was conducted to determine the progression free survival of patients treated with Crizotinib in comparison with those treated with Docetaxel or Premetrexed. The trial with an estimated enrolment of 318 patients was initiated in September 2009 and completed in June 2012. Patients from the chemotherapy arm of Phase III 1007 trial also had an option to be administered with crizotinib in the Phase II PROFILE 1005 study.
The results of the study showed that Xalkori met the primary endpoint of the study which included improving the PFS in NSCLC patients. The drug doubled the PFS to a median of 7.7 months.