Xipere™ (triamcinolone acetonide) is a corticosteroid that is delivered through suprachoroidal injection to treat macular oedema associated with uveitis, a type of eye inflammation.
Xipere is developed by US-based biopharmaceutical company Clearside Biomedical. In October 2019, the company licenced Bausch Health exclusive rights to market and develop Xipere in the US and Canada. Bausch Health also holds exclusive options to market and develop Xipere in the European Union, the UK, Australia, New Zealand, South America and Mexico.
Chinese speciality ophthalmology company Arctic Vision holds an exclusive licence to develop and market Xipere in Greater China, South Korea, Australia, New Zealand, India and the Association of South-East Asian Nations (ASEAN) countries, which include Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand and Vietnam. The company also has the right to develop and market Xipere for other ophthalmic indications in Greater China and South Korea.
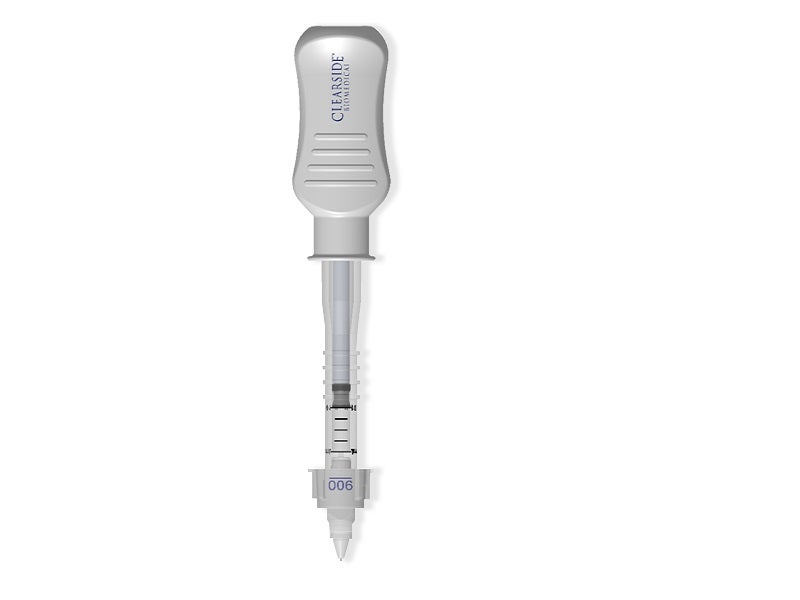
Xipere is available in a single-dose vial as a sterile, preservative-free, injectable suspension of 40mg triamcinolone acetonide formulated for suprachoroidal administration, using Clearside’s proprietary suprachoroidal space (SCS) Microinjector®.
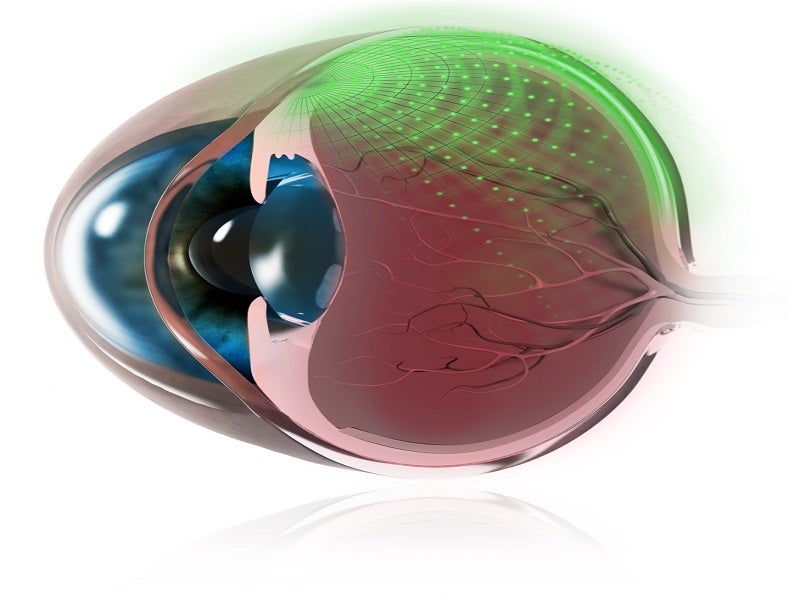
The SCS Microinjector is designed to provide unrivalled access and high bioavailability to the back of the eye, which is a common site of sight-threatening diseases. The suprachoroidal administration facilitates targeted delivery of the drug to the retina and choroid at the back of the eye.
Regulatory approvals for Xipere
In December 2018, Clearside submitted a new drug application (NDA) for Xipere to the US Food and Drug Administration (FDA) for the treatment of macular oedema associated with uveitis. The application was accepted for review in February 2019.
In August 2019, Clearside submitted an update on the NDA to the FDA concerning the stability data for Xipere manufactured using the company’s ‘enhanced’ manufacturing process. The company received a complete response letter from the FDA in October 2019 requesting additional stability data on the manufacturing process and additional data on the clinical use of the SCS Microinjector. The FDA found no issues with the drug’s efficacy studies.
In response to the FDA’s CRL, the company resubmitted its NDA for Xipere in May 2021, which was accepted for review the following month. The FDA approved Xipere for the indication in October 2021.
Causes and symptoms of macular oedema associated with uveitis
Uveitis is a type of inflammatory ocular disorder that affects the uvea, the middle part of the eye. The disease impacts around 350,000 individuals in the US and more than a million worldwide.
Uveitic macular oedema is a complication related to acute or chronic uveitis characterised by the accumulation of fluid in the macula, the part of the retina responsible for sharp, straight-ahead vision. The condition affects around one-third of all uveitis patients.
Macular oedema causes retinal enlargement and impaired vision, which can lead to irreversible vision loss if left untreated. It is the major cause of vision loss in uveitis patients and can result from any anatomic site of uveitis, including anterior, intermediate, posterior or panuveitis.
The oedema may be asymptomatic in the early stages with no visual disruption, while symptoms include a loss of contrast sensitivity, reading difficulty, metamorphopsia, micropsia and a positive relative scotoma.
Xipere’s mechanism of action
Xipere is a synthetic glucocorticoid (often called corticosteroids) with immunosuppressive and anti-inflammatory properties. Xipere primarily functions as a corticosteroid hormone receptor agonist.
Corticosteroids such as Xipere inhibit phospholipase A2 on cell membranes, preventing the breakdown of leukocyte lysosomal membranes and the formation of arachidonic acid.
The decrease in arachidonic acid reduces the expression of cyclooxygenase and lipoxygenase, inhibiting the synthesis of prostaglandins and leukotrienes.
Anti-inflammatory action is achieved by reversing vascular dilatation and decreasing permeability, thus preventing macrophage and leukocyte migration. Xipere also inhibits nuclear factor kappa-B, reducing the synthesis of pro-inflammatory signals such as interleukin-6, interleukin-8, and monocyte chemoattractant protein-1.
Clinical trials on Xipere
The FDA’s approval for Xipere was based on the positive outcomes of a randomised, multi-centre, double-masked, sham-controlled, pivotal, Phase III clinical trial named PEACHTREE.
The trial enrolled 160 patients with macular oedema associated with uveitis, who were randomly assigned to receive either two 4mg Xipere via suprachoroidal injection or two sham injections, 12 weeks apart.
The study’s primary measure outcome was the percentage of patients whose best-corrected visual acuity (BCVA) had improved by at least 15 letters from baseline after 24 weeks of follow-up.
At week 24, 47% of the patients treated with Xipere improved their BCVA by at least 15 letters, as measured by the Early Treatment of Diabetic Retinopathy Study Scale, compared to 16% of the patients in the control arm.
The most common adverse reactions observed in patients with Xipere during the clinical study were elevated intraocular pressure and eye pain.
Besides PEACHTREE, the clinical programme evaluating Xipere’s safety and efficacy also included a non-interventional extension study, MAGNOLIA, and an open-label safety trial, AZALEA.