Developed by Novo Nordisk, Xultophy 100/3.6 is indicated for the treatment of type 2 diabetes.
A new drug application was submitted to the US Food and Drug Administration (FDA) on 12 September 2016 and the drug was approved on 21 November 2016.
Xultophy 100/3.6 will be launched in the US in the first half of 2017.
In addition, it was first approved in Switzerland on 12 September 2014 and the European Commission on 18 September 2014.
Type 2 Diabetes causes and symptoms
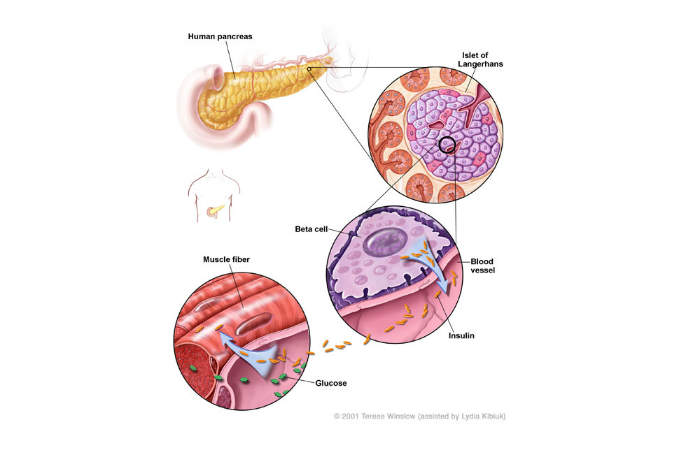
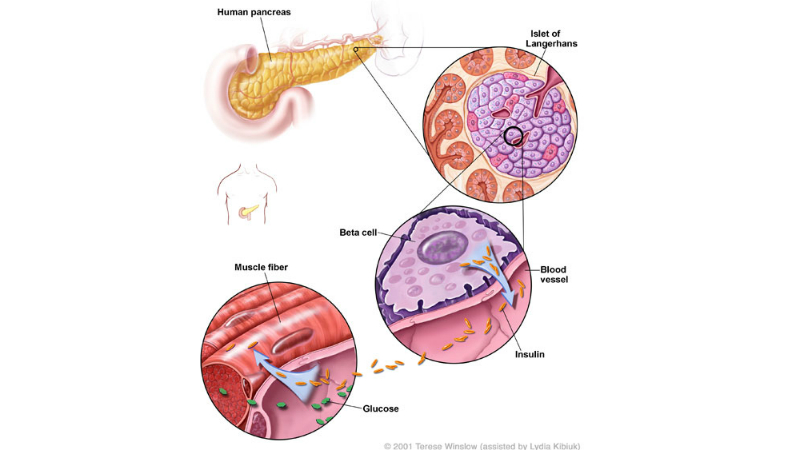
Diabetes is a metabolic disorder characterised by increased blood sugar levels, insulin resistance, or lack of insulin in the body. It is usually classed as type 1 or type 2.
Type 1 diabetes mostly occurs in children and rarely in adults. In this condition, the body does not make enough insulin to maintain low blood sugar levels.
Type 2 diabetes is the most common type and it occurs due to a lack of insulin production. The condition initially begins with resistance to insulin and mostly occurs in middle-aged people and the elderly.
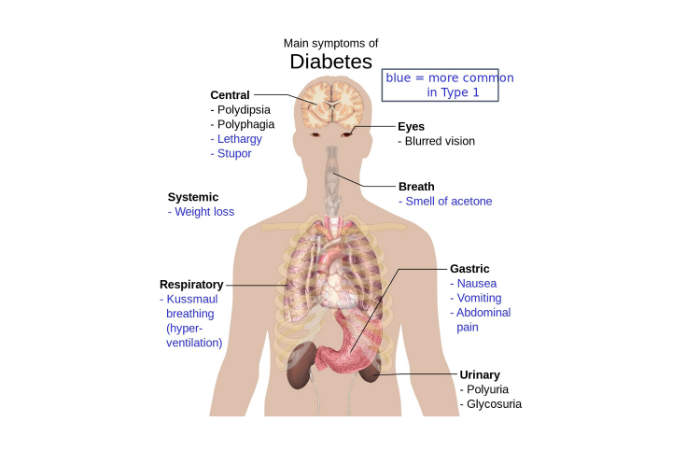
The symptoms associated with type 2 diabetes include increased thirst, frequent urination, unusual weight loss, blurred vision, fatigue, sores, and increased appetite. In the long-term, the disease may lead to serious conditions such as heart diseases, diabetic retinopathy, and kidney failure.
Xultophy mechanism of action
Composed of insulin degludec and liraglutide, Xultophy 100/3.6 is available in the form of an injectable solution.
Insulin degludec is a basal human insulin analog, which plays an important role in regulating glucose metabolism through the stimulation of glucose uptake by skeletal muscles and fat. It also further inhibits hepatic glucose production to lower blood glucose levels, inhibits lipolysis and proteolysis, and improves protein synthesis.
Liraglutide is a GLP-1 (Glucagon Like Peptide -1) receptor agonist, which acts by increasing the release of glucose-dependent insulin.
Clinical trials on Xultophy
The approval of Xultophy 100/3.6 was based on results obtained from the three phase III clinical trials, Study A, Study B, and Study C, which was conducted on a combined 1,393 patients to examine the safety and efficacy of the drug.
Study A was a phase III, randomised, open-label, 26-week trial, which enrolled 348 subjects with type 2 diabetes who were already being treated with liraglutide and metformin alone, in combination with pioglitazoneor sulfonylurea, or both. The subjects continued to receive oral anti-diabetic drugs as pre-trial doses and throughout the trial.
The subjects were randomised to receive 16 units of Xultophy 100/3.6 or 1.7mg liraglutide as starting doses. The trial met the primary endpoint of change in HbA1C levels from the baseline and demonstrated the superiority of the drug (1.31%) compared to liraglutide (0.36%).
Study B was a phase III, randomised, double-blind, 26-week trial conducted on 398 patients with type 2 diabetes already treated with basal insulin and metformin or as a combination with sulfonylurea or glinides.
The subjects were randomised to receive 16 units of Xultophy 100/3.6 or 16 units of insulin degludec as starting doses. The trial met the primary endpoint of change in HbA1C levels from the baseline and demonstrated the superiority of the drug (1.94%) compared to insulin degludec (1.05%).
Study C was a phase III, randomised, open-label, two-arm, parallel trial, conducted on 557 patients with type 2 diabetes who already received insulin glargine U-100 and metformin.
The subjects were randomised to receive 16 units of Xultophy 100/3.6 or 32 units of insulin glargine U-100 as starting doses. The trial met the primary endpoint of change in HbA1C levels from the baseline and demonstrated the superiority of the drug (1.67%) compared to insulin glargine U-100 (1.16%).