Zegerid is a proton pump inhibitor (PPI) comprising omeprazole, a leading prescription acid that reduces heartburn and sodium bicarbonate, an antacid.
The drug is indicated for the treatment of duodenal ulcer, gastric ulcer, gastroesophageal reflux disease (GERD) and erosive oesophagitis. Zegerid was developed by Santarus and is licensed by Merck.
In December 2009, Merck and Santarus announced that over-the-counter version of Zegerid was approved by the US Food and Drug Administration (FDA). Santarus submitted a New Drug Application (NDA) for Zegerid OTC in March 2008.
The Zegerid OTC became available in the US market in April 2010.
Zegerid OTC is being marketed by Schering-Plough Health Care Products, a consumer healthcare division of Merck. The companies merged in November 2009.
Heartburn causes and associated conditions
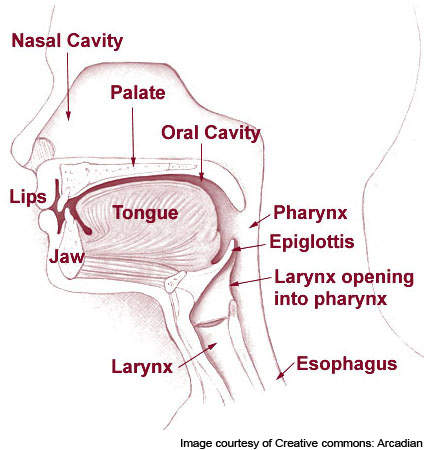
Heartburn occurs when stomach acid refluxes or runs back into the oesophagus, resulting in a painful burning sensation in the chest or throat. When food is consumed it travels from the mouth to the stomach through the oesophagus.
Here stomach acid starts to break down the food. In a person suffering from heartburn the stomach acid begins to flow back to the throat or chest through the oesophagus.
If the instance of heartburn is more than twice a week, it is categorised as GERD. In people suffering from GERD, the muscles at the end of the oesophagus do not close completely. As a result the contents of the stomach flow back, or reflux, into the oesophagus and irritate it.
There are various causes of heartburn such as pregnancy, spicy foods, alcohol and a few medications. Heartburn needs to be treated appropriately to avoid damage to the oesophagus.
Zegerid – proton pump inhibitor for heartburn treatment
Zegerid is the first and the only PPI in the market for the treatment of heartburn. It works by inhibiting gastric acid secretion for 24 hours. Studies have shown that PPIs are the most effective method of treating acid reflux disease.
The omeprazole contained in Zegerid is a crystalline powder that dissolves with decomposition at about 155°C. The stability of omeprazole is dependent on pH, so it quickly degrades in acid media. Sodium bicarbonate is therefore added to Zegerid to increase the gastric pH and protect omeprazole from acid degradation.
Zegerid is available in the form of immediate-release capsules, chewable tablets and unit-dose packets as powder for oral suspension. Zegerid is most effective in patients suffering from nocturnal acid breakthrough or for individuals who require immediate relief.
The FDA approval for marketing the OTC version of Zegerid is another addition to the Zegerid family of branded prescription drugs. Zegerid capsules are already approved by the FDA. Santarus submitted an NDA for Zegerid capsules to the FDA in April 2005. The FDA approved the application in February 2006.
Santarus is planning to further expand the formulations of Zegerid. In January 2009, Santarus submitted an NDA to the FDA for marketing Zegerid in swallowable tablet formulation. The new formulation is an immediate-release tablet consisting of omeprazole and a mix of buffers.
Clinical trials show efficacy in various indications
Clinical trials with omeprazole have been carried out for various indications. These include active duodenal ulcer, gastric ulcer, GERD and erosive oesophagitis. The studies revealed that omeprazole was well tolerated and provided significantly faster and complete relief from the respective indications.
Zegerid has also been studied in the reduction of risk of upper gastrointestinal bleeding in critically ill patients. The study revealed that Zegerid helped in reducing the risk of upper GI bleeding.
A head-to-head clinical study completed in May 2010 proved that Zegerid OTC reduced and controlled acid levels much better than Prevacid 24HR. The study was intended to evaluate the speed and acid reduction on the seventh day of the medication.
Zegerid reduced the acid level to clinically accepted gastric pH levels twice as fast as Prevacid 24HR.
Marketing commentary
With the FDA approval of Zegerid OTC, the market position of the drug will significantly improve. Zegerid OTC is expected to pose strong competition to Procter & Gamble’s Prilosec OTC (omeprazole 20mg) and Novartis’ Prevacid 24HR (lansoprazole 15mg).
Zegerid OTC will also provide a significant boon to both Merck and Santarus. The FDA approval of Zegerid will give Santarus a $20m milestone payment and another $37.5m in sales milestone payments and royalties on sales.