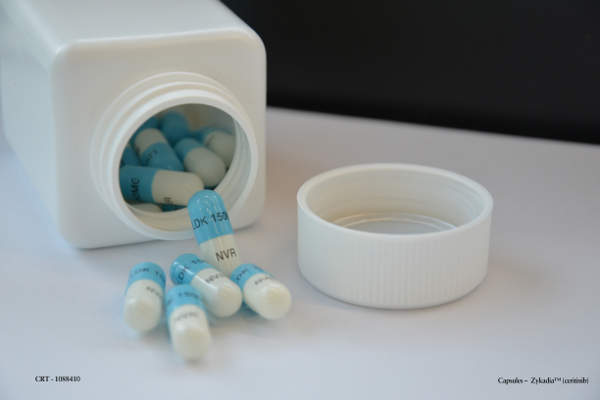
Zykadia (Ceritinib / LDK378) is an inhibitor of anaplastic lymphoma kinase (ALK) indicated for treatment of non-small cell lung cancer (NSCLC).
The drug was discovered and developed by Novartis Pharmaceuticals and approved by the US Food and Drug Administration (FDA) for treating ALK-positive metastatic NSCLC patients following treatment with Crizotinib in April 2014.
The Committee for Medicinal Products for Human Use (CHMP) of EMA adopted positive opinion for granting conditional marketing authorisation for Zykadia for the treatment of adult patients with ALK positive advanced NSCLC in February 2015. In May 2015, the European Commission (EC) approved the drug for the same indication in the EU.
In May 2017, the FDA approved the expanded use of Zykadia (ceritinib) in the first line treatment of patients suffering from ALK-positive metastatic NSCLC.
Non-small cell lung cancer drug
Gilotrif (afatinib) is an orally administered drug for the first-line treatment for metastatic non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations.
Non-small cell lung cancer (NSCLC) is the most common type of cancer. It accounts for approximately 85% of all lung cancers and is the leading cause of cancer-related deaths worldwide.
The disease occurs when abnormal cells rapidly multiply and do not stop reproducing.
Zykadia mechanism of action
Zykadia contains an ALK inhibitor that works against the ALK gene involved in development of cancers. The drug is available in 150mg gelatin capsules for oral administration.
Clinical trials on Zykadia
Phase II clinical trials on Zykadia were conducted between July 2005 and October 2007. The open label, non-randomised, parallel assignment enrolled 85 patients with NSCLC.
The primary outcome measure was clinical efficacy based on the evaluation of objective tumour response rate (RR). The secondary outcome measures included assessment of the safety of RAD001 monotherapy, additional clinical efficacy of RAD001 and the steady state levels of RAD001 in blood, and to investigate potential molecular markers predictive of clinical effect.
Novartis initiated another Phase II clinical trial on Zykadia in April 2012. The open label, multi-centre, interventional and parallel assignment enrolled 83 patients.
The primary outcome measure of this study was frequency and characteristics of dose limiting toxicities (DLT) in 28 days, whereas the secondary outcome measures included overall survival (OS), frequency, duration and severity of adverse events (AE), progression-free survival (PFS), and plasma concentration of INC280.
FDA approval for Zykadia was based on results obtained from a pivotal phase III clinical trial. It was a single-arm, open-label study that enrolled 163 subjects with metastatic ALK-positive NSCLC, who progressed while receiving or were intolerant to Crizotinib.
Patients were administered with Zykadia at a dose of 750mg once-daily. The study enrolled patients with a median age of 52 years. The most common sites of metastases evaluated in the clinical study included brain, liver, and bone.
Results of the study demonstrated that the patients who were treated with Zykadia achieved an overall response rate (ORR) of 54.6% and a median duration of response (DOR) of 7.4 months.
The most common adverse reaction found in the Zykadia-administered patients included diarrhoea, nausea, elevated transaminases, vomiting, abdominal pain, fatigue, decreased appetite, and constipation.
Safety and efficacy of Zykadia were evaluated in Study 1, which enrolled 304 patients. Of these, 255 were treated with Zykadia doses ranging between 50mg and 750mg.
The results of the study demonstrated that approximately 96% of the 255 patients who were treated with Zykadia experienced diarrhoea, nausea, vomiting or abdominal pain. The study also found that drug-induced hepatotoxicity occurred in patients treated with Zykadia.
Study 1 further found that around 3% of the 255 patients experienced a QT interval (QTc) increase over baseline greater than 60msec. Pneumonitis was reported in 4% of 255 patients treated with Zykadia, while sinus bradycardia occurred in 1% of 255 patients treated with Zykadia.
The EC’s approval of Zykadia was based on the data obtained from two clinical trials known as ASCEND-1 and ASCEND-2, which were open-label, single-arm, global and multicentre studies. The ASCEND-1 study enrolled 246 ALK+NSCLC patients, whereas the ASCEND-2 study enrolled 140 patients with locally advanced or metastatic ALK+ NSCLC.
Results of the ASCEND-1 demonstrated that the patients who were administered with Zykadia 750mg daily after previous treatment with chemotherapy, followed by an ALK inhibitor, experienced ORR of 56.4%, the median DOR was 8.3 months, and the median PFS was 6.9 months.
The most common adverse reactions found in both of the clinical studies in the patients treated with Zykadia included diarrhoea, nausea, vomiting, tiredness, abdominal pain, decreased appetite, constipation, rash, heartburn and anaemia.
Marketing commentary
Zykadia was approved under the FDA’s accelerated approval programme. The drug is marketed worldwide by Novartis. Other medications approved for the same indication include Gilotrif (afatinib) developed by Boehringer Ingelheim Pharmaceuticals and Xalkori manufactured by Pfizer.