No Filter Selected
No Filter Selected
No Filter Selected
No Filter Selected

ALS: Clene plans 300-patient Phase III trial of CNM-Au8
CNM-Au8 clinical advisor Matthew Kiernan previews Clene’s planned Phase III ALS trial design, including biomarkers and target population.

Cytokinetics terminates Phase III program in ALS
Cytokinetics will discontinue its Phase III program of reldesemtiv in ALS after the COURAGE-ALS study met criteria for futility.

AL-S Pharma’s ALS mAb meets primary endpoint in Phase II trial
AL-S Pharma said the trial met the primary endpoints and showed a clinically meaningful change in secondary endpoints.
NeuroSense makes key progress in Phase II ALS trial
NeuroSense is investigating PrimeC in a Phase IIb trial targeting ALS as the company looks to compete in a crowded drug development field.

ALS Therapy Development Institute develops clinical trial mapping tool
The ALS Trial Navigator helps patients and researchers find relevant amyotrophic lateral sclerosis (ALS) clinical trials worldwide.

NeuroSense previews Phase IIb trial plans for oral ALS drug
NeuroSense CEO Alon Ben-Noon reveals the target patient population and outcome measures of a planned Phase IIb ALS trial to Clinical Trials Arena.
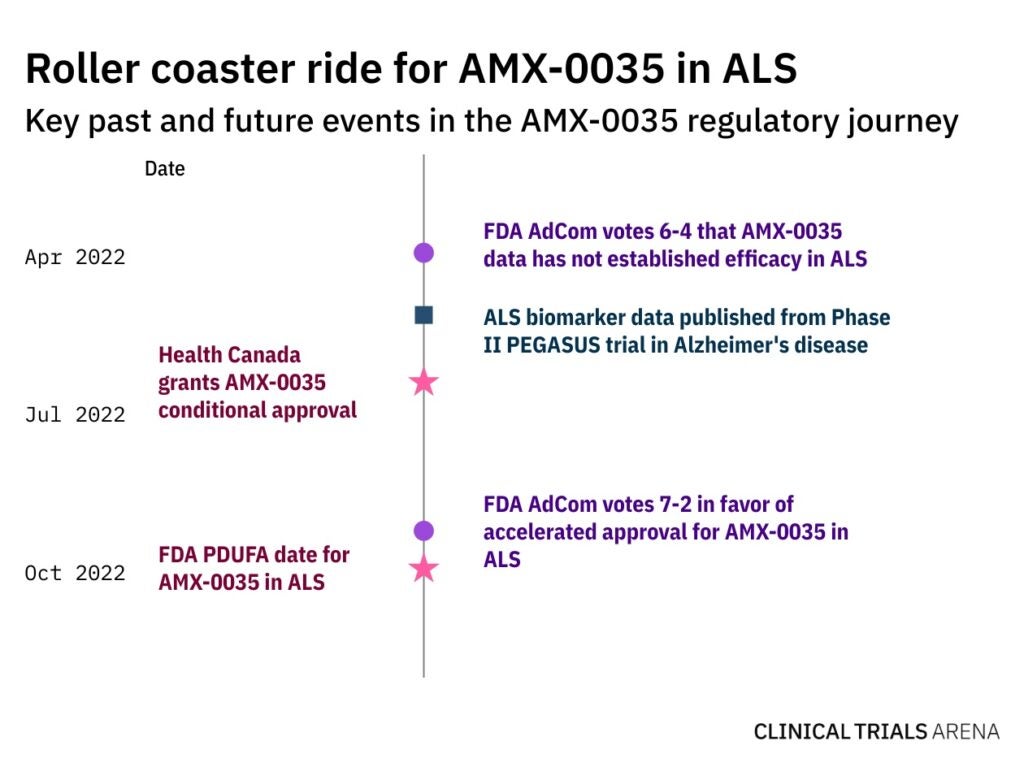
Looming Amylyx drug approval decision renews debate over ALS endpoints
With the FDA's decision on Amylyx’s AMX-0035 fast approaching, we dive into tensions over ALS endpoints and major drug trials to watch.
FDA lifts clinical hold on Neurizon’s ALS drug
The lifting of the FDA’s clinical hold on Neurizon’s drug will pave the way for its investigation in the HEALEY-ALS trial.

New biomarker data supports NeuroSense’s ALS drug
NeuroSense’s ALS drug Prime C regulated iron levels, which are thought to be associated with improved survival rates and disease mitigation.

FDA to hold Advisory Committee meeting for BrainStorm’s NurOwn in ALS
In a surprising about-face, the FDA will convene an Advisory Committee to review BrainStorm Cell Therapeutics’s stem cell therapy NurOwn for ALS.

Results of HEALEY ALS trials show progress in the field
There are seven regimens in HEALEY ALS Platform Trials, with five completed and two trials recruiting patients at Phase III.

ProJenX and Unlearn partner to use AI-generated digital twins in ALS trial
The partnership will employ the ALS-Digital Twin Generator of Unlearn to enhance the trial's evaluation process.

AL-S takes mAb to Phase III after mid-stage ALS success
If approved, AP-101 could rival Biogen and Ionis’ Qalsody due to its more convenient route of administration.

Medidata and Project ALS partner to develop new therapeutic strategies
Medidata, a subsidiary of Dassault Systèmes, and Project ALS have entered a research partnership in order to develop new therapeutic strategies for the treatment of Amyotrophic lateral sclerosis (ALS).

AI Therapeutics reports promising Phase II data in ALS
AI Therapeutics reported positive data in a small trial targeting a genetic form of ALS, setting the stage for possible further development.

NeuroSense taps PhaseV’s ML tech for Phase III ALS trial analysis
PhaseV will analyse NeuroSense’s Phase II ALS trial data, and results from these will inform Phase III trial design, enrolment, and cost-effectiveness.
Ionis begins clinical trial of antisense medicine for ALS treatment
Ionis Pharmaceuticals has initiated a Phase III clinical trial of antisense medicine ION363 in patients with amyotrophic lateral sclerosis (ALS) with mutations in the fused in sarcoma gene (FUS).

FDA AdCom overwhelmingly supports accelerated approval for Biogen’s tofersen in ALS
AdCom panelists backed an accelerated approval for Biogen’s tofersen in a rare form of ALS based on a new surrogate biomarker.
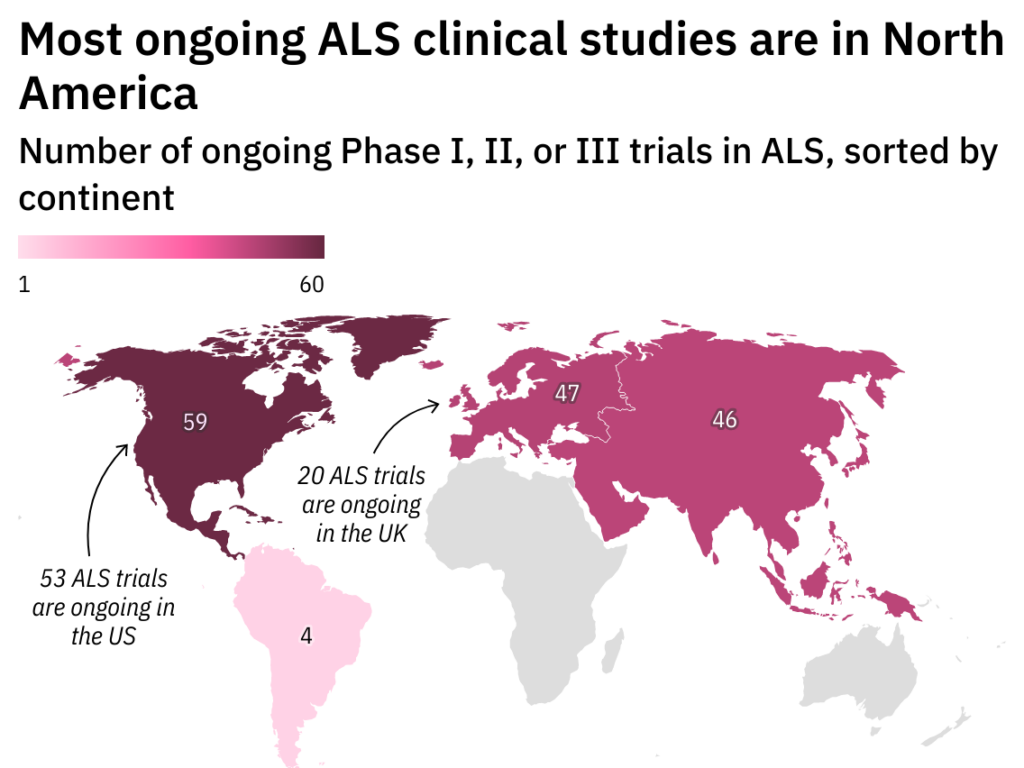
Mapping Endpoints: How to build an ALS clinical trial
Differing endpoint and efficacy expectations can make designing an ALS clinical trial challenging. Clinical Trials Arena looks at ways to dampen drug development obstacles.
Amylyx doses first subjects in Phase III ALS treatment trial
The PHOENIX trial will analyse the safety and efficacy of AMX0035 in nearly 600 patients enrolled at 65 centres in Europe and the US.
