
The pharmaceutical industry is undergoing a major transformation, as clinical trials become increasingly data heavy and digital-based.
Traditional methods of data collection are gradually being replaced by sophisticated digital solutions. Among the most significant advancements are electronic Patient-Reported Outcomes (ePRO) and electronic Clinical Outcome Assessments (eCOA), which have fundamentally reshaped how patient data is collected, analyzed, and used in clinical trials.

According to GlobalData’s report, Thematic Intelligence: Digital Transformation and Emerging Technologies in the Healthcare Industry 2023 Edition, 71% of surveyed healthcare industry professionals believed that the Covid-19 pandemic had been the most impactful event on digital transformation in the healthcare sector in recent years. The pandemic accelerated the rise of remote patient monitoring (RPM) and decentralized clinical trials (DCTs), as well as cloud-based technologies. In fact, the eCOA market is valued at over $1bn annually and is growing at a rate of more than 15% a year.
While the adoption of ePRO and eCOA systems is on the rise, not all solutions are created equal. Differences in functionality, adaptability, and user experience can significantly impact the outcome of a clinical trial.
For sponsors, choosing the right system is crucial for the success of their studies. Consequently, a negative experience with a digital platform can make sponsors revert to paper-based methods. Yet it doesn’t have to be this way.
Why customization matters in ePRO and eCOA
One of the key differentiators in the ePRO and eCOA market is the level of customization that a solution offers. Standardized platforms may provide a baseline of functionality, but they often fall short in trials that require more specialist data collection. This is particularly true in studies involving rare diseases or complex patient populations, where a one-size-fits-all approach can lead to suboptimal data and even the failure of the trial.
Datacubed Health has recognized this challenge by developing solutions tailored to the specific needs of each clinical trial. By collaborating closely with sponsors, the company ensures that its systems are not only compliant with regulatory requirements but also designed to capture the most relevant and accurate data.
One of the company’s innovative tools is the posture diary, a unique feature that allows a patient’s physical posture to be monitored over time. This is particularly useful in studies where physical activity or mobility is a key outcome measure. By providing this level of detail, posture diaries can offer insights that might be missed with traditional data collection methods.
Similarly, Datacubed Health’s customized food diaries are changing how dietary data is collected in clinical trials. Rather than relying on generic food tracking tools, which can be imprecise and difficult for patients to use, these customized diaries are designed to capture detailed and relevant dietary information. This is crucial in studies where nutrition plays a significant role in patient outcomes, such as those involving metabolic disorders or weight management interventions.
Real-world applications: transforming clinical research
The benefits of these innovations are proven in real-world clinical trials. In collaboration with researchers from the University of California, San Francisco (UCSF), Datacubed Health’s cognitive testing tools have been instrumental in diagnosing Frontotemporal dementia. This condition is often misdiagnosed due to its complex presentation and requires nuanced and precise data to ensure an accurate diagnosis.
Datacubed Health’s eCOA tools have provided the level of detail needed to make these diagnoses more reliable, demonstrating the transformative impact that advanced digital tools can have on clinical research.
The future of ePRO and eCOA in clinical trials
Remote and hybrid trials, as well as virtual data collection, are rapidly becoming the norm rather than the exception. According to GlobalData’s 2023 Digital Transformation and Emerging Technologies survey, the virtual components most used in DCTs include remote patient monitoring and telemedicine (39%), wearables (26%, web-based technologies such as eCOA and ePRO (25%), mobile tech and smart devices (18%), as well as in-home devices such as blood pressure monitors and oximeters (15%).
The role of ePRO and eCOA systems is becoming even more important. These tools enable the collection of real-time data from patients, regardless of their location, making it easier to conduct remote trials without sacrificing data quality. However, as the market for these solutions expands, so too does the need for differentiation. Sponsors will increasingly seek out providers who can offer more than just a standard platform.
However, as more data is collected digitally, the risk of data breaches and cyberattacks increases. Sponsors and platform providers must ensure that their systems are compliant with data protection regulations such as GDPR and must have robust security measures in place to protect patient data.
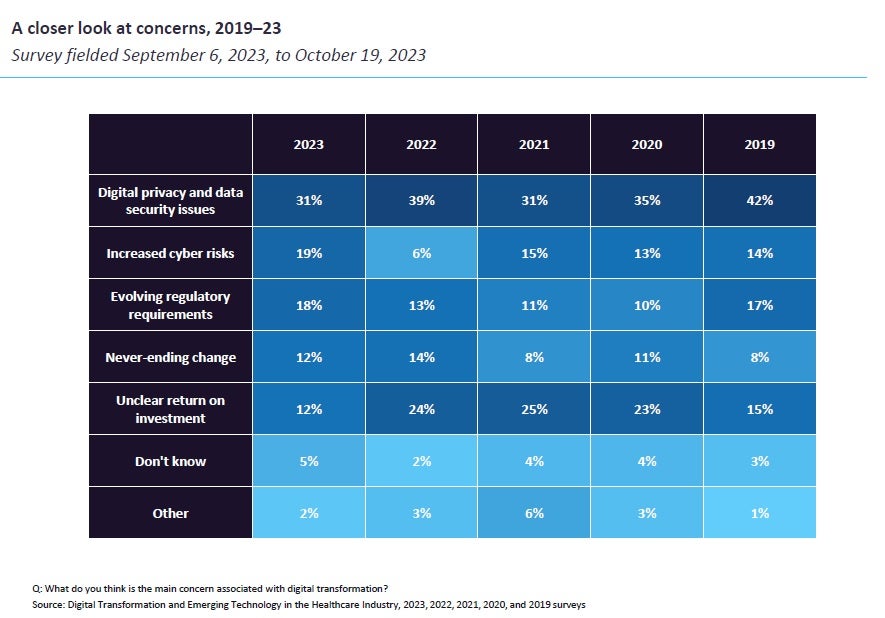
Datacubed Health considers cybersecurity of paramount importance, ensuring that technological innovations within clinical trials are underpinned by robust security measures. The company focuses on compliance with strict regulatory standards such as GDPR and HIPAA, which are essential for protecting patient data during trials. This compliance ensures that patient privacy is maintained across all stages of data collection and management.
To safeguard sensitive information, Datacubed integrates advanced security protocols within its platforms, including features such as audit trails and secure data storage. The company’s use of behavioral science not only enhances patient engagement but also strengthens data protection by ensuring that security measures align with user behavior, reducing the likelihood of breaches due to human error.
By focusing on customization, real-world applications, and patient engagement, Datacubed Health is helping to shape the future of clinical research. To find out more, register here for the company’s forthcoming webinar.


