S-CLINICA
CTMS Vendor for Complex Clinical Trials
S-CLINICA is a trusted clinical trial management system (CTMS) vendor for complex clinical trials, providing expedite integrated response technology (IRT) support for Covid-19 clinical trials.
Subscribed
You have successfully submitted your enquiry. Someone from our company will respond ASAP
About Us

S-CLINICA is a trusted clinical trial management system (CTMS) vendor for complex clinical trials, providing expedite integrated response technology (IRT) support for Covid-19 clinical trials.
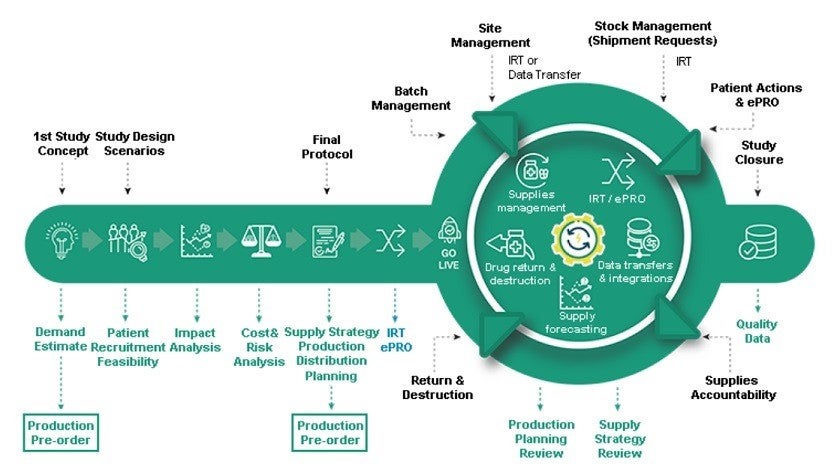
The company supplies a single platform offering Clinical Supply Forecasting, Cost and Risk Optimisation, Interactive Web Response System (IWRS), Clinical Supply Management, Drug Return and Destruction, Data Transfer & Integration.
CTMS vendor for complex clinical trials
Numerous pharmaceutical, biotechnology, and contract research organisation (CRO) companies select S-CLINICA as their preferred IRT vendor. With more than 900 successful clinical studies across 116 countries, the company delivers fully customisable and integrated solutions, having done so for 23 years in various therapeutic areas and study phases.
With the growing complexity of clinical trial design, S-CLINICA pays undivided attention to the employment of innovative technology solutions based on a science-driven approach, as well as proactive project management.
CTMS for clinical trial anticipatory management
S-CLINICA’s ClinVision is an innovative CTMS for clinical trial anticipatory management, the best-in-class single platform uniting Data Transfer & Integration, Drug Return and Destruction, Clinical Supply Management, IWR, Cost and Risk Optimisation, and Clinical Supply Forecasting.
CTMS Clinical Supply Module assists with estimating the demand in supplies from the first study concept stage when least information is accessible, allowing the company to recognise the best supply strategy and prepare robust supply production and distribution.
ClinVision assists in anticipating and mitigating risks, keeping costs under control. The platform offers an analysis of study pitfalls and impact from changes in study assumptions, as well as protocol design.
IRT system for clinical study live stage
Users can harness real-time data obtained from advanced and unique built-in, fully flexible IRT system during the live stage of a clinical study, deployed within short timelines for a trial of any complexity.
Furthermore, operators can update the distribution plan for medical kits, as well as develop the subsequent production campaigns, using the intelligent and pro-active alerts system to enhance the project management of a clinical trial.
Preventing unforeseen issues
ClinVision can review the influence on production planning and costs, even with low recruitment or high screen failure rate. Possible changes in protocol, and supply management changes and potential production delays, can be evaluated in safe mode without modifying the production process.
Study parameters can be easily changed and updated, with clean study data delivered in an hour, at any stage of the clinical trial.
About S-CLINICA
Founded in 1997, S-CLINICA dedicates itself to ensuring each study is a success. For more than two decades, the company offers a dynamic adoption of scientifically proven practices, excelling in delivering fully integrated and customisable solutions.
The company offers immediate support, as well as meticulous attention, to each project from start to finish, building long-term relationships with each client and its team.
S-CLINICA remains the preferred partner of its first-ever client, a Top-5 Pharma Biotech Company, which resulted in 23 years of successful collaboration.
Contact Details
Website
Email Address
Address
1050 Brussels,
Belgium