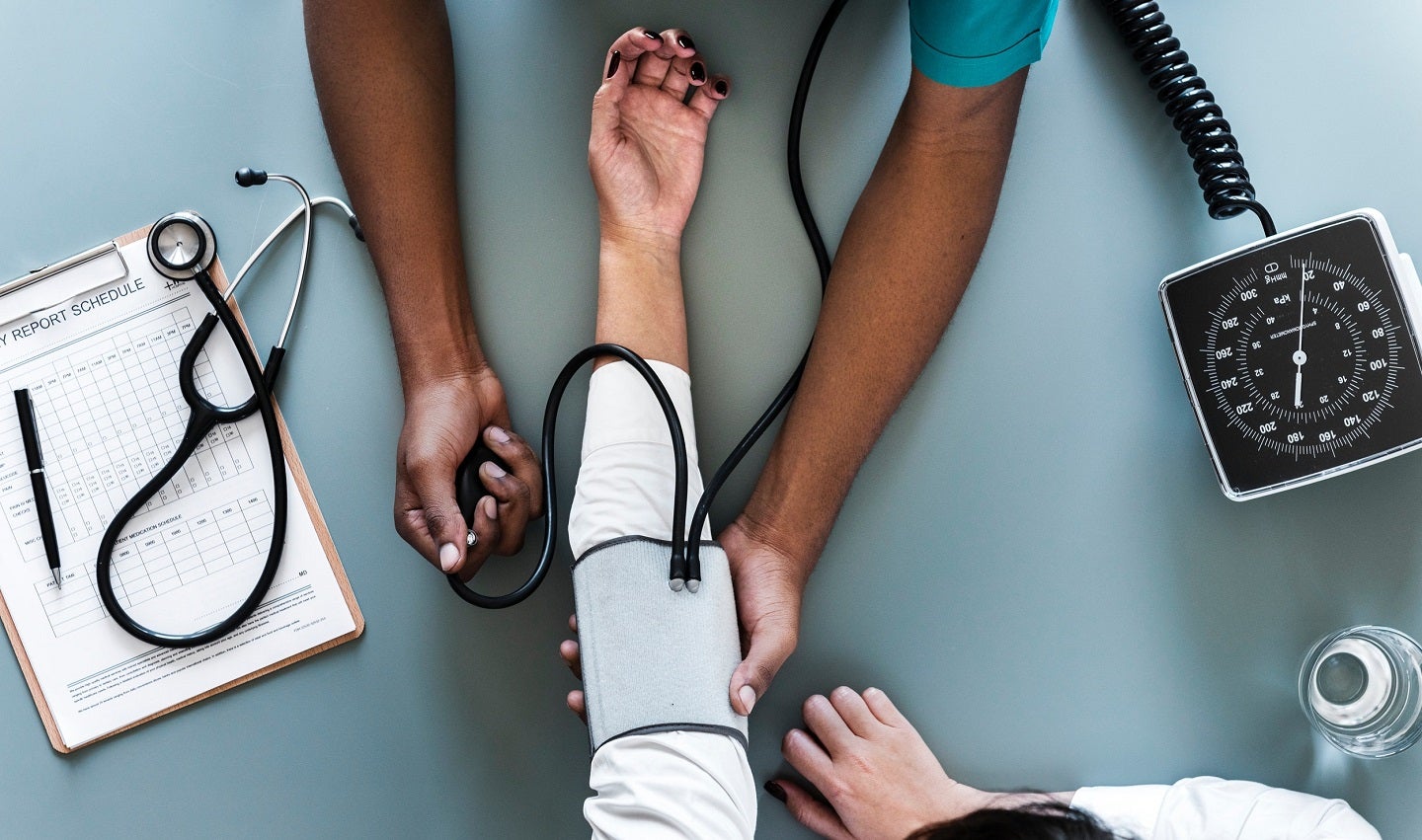
Mineralys Therapeutics has reported positive results from the Target-HTN Phase II clinical trial of lorundrostat to treat uncontrolled and resistant hypertension.
The double-blind, randomised, placebo-controlled, dose-ranging, multicentre Target-HTN trial was conducted in the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It assessed the efficacy, safety and tolerability of orally administered lorundrostat on blood pressure (BP) to treat hypertension.
The therapy was evaluated when used as an add-on therapy to stable background treatment of two or more antihypertensive agents in 200 male and female subjects, aged 18 years or above.
In the Phase II trial, five active doses of lorundrostat (12.5mg QD, 50mg QD, 100mg QD, 12.5mg twice daily [BID], and 25mg BID) were compared to a placebo in hypertensive subjects.
A modest rise in serum potassium, and a decrease in estimated glomerular filtration rate, urinary tract infection, and hypertension were the adverse events that were observed. One serious adverse event reported was possibly related to the study drug was hyponatremia.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial demonstrated a clinically meaningful reduction in blood pressure with once-daily dosing of lorundrostat.
Mineralys chief medical officer David Rodman said: “Our hypothesis when starting Target-HTN was that obesity, abnormal aldosterone biology, and hypertension were linked intrinsically.
“In finding that lorundrostat demonstrated clinically meaningful improvement in obese individuals, we’ve validated our initial thinking, and now have data that will be crucial for developing future trials and bringing lorundrostat to market.”
Based on the preliminary topline results from the Target-HTN trial, it was found that treatment with lorundrostat at 50mg and 100mg doses once daily (QD) led to a statistically significant systolic BP reduction in inadequately controlled hypertensive patients on a minimum of two background antihypertensive medications.





