 Oximio is a leading supplier of end-to-end clinical trial services, bringing experience and expertise to the global clinical research industry. The company employs more than 300 highly skilled colleagues across multiple countries, delivering quality customer service to ensure the highest operational standards.
Oximio is a leading supplier of end-to-end clinical trial services, bringing experience and expertise to the global clinical research industry. The company employs more than 300 highly skilled colleagues across multiple countries, delivering quality customer service to ensure the highest operational standards.
Clinical trial project management services
Oximio provides a single point of contact for all clinical trial projects, offering project management services from start to finish. Before beginning any project, our dedicated project managers will develop a logistics strategy that takes the product profile and any region-specific import requirements into consideration to ensure the trial can run smoothly, safely and cost-effectively.
Our biosample management service exports and delivers clinical trial samples to central laboratories, while specimens are collected by our fleet of temperature control vehicles and insulated shapers. If required, our team can consolidate samples at the depot. We take care of all airport and customs procedures in the country of collection and destination so they can be forwarded to the central laboratory.
Oximio has a large and loyal clientele working with global pharmaceutical companies, contract research organisations (CROs), and contract manufacturing organisations (CMOs). The company is constantly expanding by introducing new services and creating more innovative services for clients.
Logistics services for the entire clinical trial process
Oximio’s comprehensive clinical trial logistic service provides an all-in-one solution for various clinical trial requirements. The service encompasses customs clearance and IOR, cold chain logistics and storage, biosample management, and the return, accountability and destruction of medicines. Our diligent project management team takes proactive measures to uphold quality protocols and effectively handle challenges, modifications, and unforeseen obstacles that may emerge throughout the trial’s execution.
Oximio can customise and enhance the clinical trial supply chain by using a range of logistical strategies, including our own GMP Depot Network and process expertise. This ensures that supplies are delivered to the correct locations on time and in the correct quantities, all while remaining within budget.
Comparator sourcing capabilities for clinical trials
Oximio’s comparator sourcing service is designed to meet customers’ timelines and budgets. We ensure regulatory compliance by adhering to and working within legal requirements, including good clinical practice (GCP) and good distribution practice (GDP). We provide comparator sourcing for both investigational and non-investigational medical products for trials, in addition to medical devices and equipment.
Our wide geographical reach enables us to source products from across Europe, the Americas, Africa, the Middle East and Asia. We can carry out objective analysis of availability and can offer competitive pricing, in addition to short lead times. Oncology is one of our most predominant clinical research areas and we can guarantee the best market price for Keytruda with a four-week lead time.
Medicine access services for clinical trial patients
Oximio’s patient travel and patient outreach services understand the needs of patients at every phase of a trial. We provide appropriate solutions to maximise patient recruitment, engagement, and retention throughout clinical trials.
Our expanded access programmes can provide patients with access to non-registered medication outside a clinical trial. We can provide risk-free, direct-to-patient delivery through air and train travel, as well as a ground transportation network of vehicles backed by fully trained and experienced drivers.
Oximio’s patient-centricity service is an easy-to-use solution that provides exclusive 24-hour customer support for patients. The fully customised service is available on demand and is entirely transparent, with its invoicing and reporting compliant with ISO-certified QMS.
Fully connected clinical trial material supply network
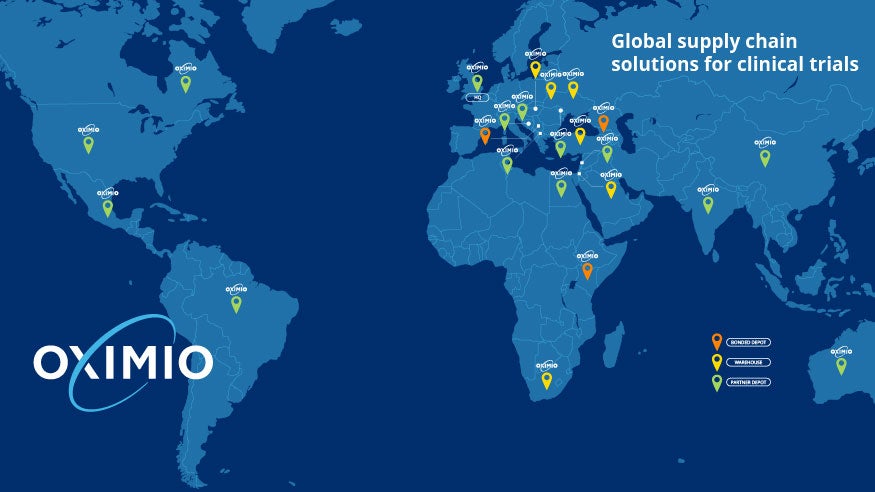
Oximio’s extensive global network of depots and bonded warehouses connects multiple patient communities of more than 500 million people in total. Our warehouse offering provides multiple solutions for clinical trials, including:
- regulatory compliance
- traceability and record-keeping
- enhanced security and compliance
- efficient logistics and inventory management
- temperature control solutions
- labelling
Combining our exceptional depot network and highly skilled, qualified team of experts, we provide extensive knowledge of both global and local procedures for clinical trials, guaranteeing the highest operational standards.
Comprehensive clinical trial management services
Oximio offers a complete end-to-end project management service with dedicated project managers, who provide full support to customers for the duration of their trial. With a three-tiered package structure, we can offer flexible pricing to accommodate customer needs.
Our project planning and execution includes risk and timeline analysis, budgeting and reporting, regulatory consulting, custom brokerage support, IOR and exporter of record (EOR) services, and guidance on import and export license/permit requirements.













