TrialHub empowers clinical research via a 360-degree feasibility suite that builds on generative AI to provide instant access to real-time, peer-reviewed scientific databases. With our AI-powered chat, epidemiology-level intelligence and detailed patient insights, clinical trial strategists can plan a trial from start to finish in a few clicks. TrialHub databases have been carefully verified by an international team of medical experts.
TrialHub IQ, our AI-based natural language search engine, transforms TrialHub into a knowledge hub for clinical research that connects to more than 80,000 data sources, as well as millions of peer-reviewed PubMed publications and medical guidelines. The service offers tools to help users identify and assess potential clinical trial sites, recruit patients, and better understand the local reimbursement landscape to help them plan successful patient-centric clinical trials.
Patient feasibility for successful recruitment and retention
TrialHub goes beyond the standard site and country feasibility data to ensure successful clinical trial recruitment. Users can benefit from deep patient understanding opportunities by performing a first-of-its-kind Patient Feasibility assessment.
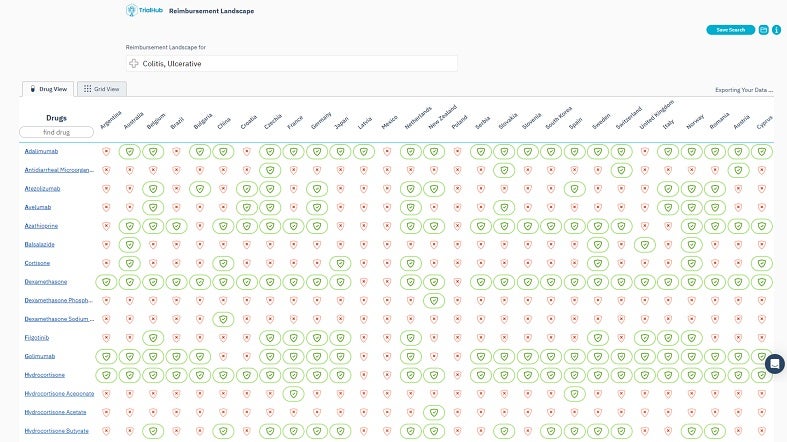
Patient Feasibility helps experts understand where their competitor drug is reimbursed and, as part of the local standard of care, locates eligible patients in countries where the patient pathway corresponds with their trial’s specific requirements. Integrating insights about reimbursement and utilising TrialHub’s AI-powered chat can effectively map out patients’ journey, illustrating their progression from initial symptoms to treatment.
In addition, TrialHub allows users to generate unique insights that are specific to their own needs by uploading their own proprietary data. For example, a user could upload their own unstructured patient data to generate insights into the feasibility of adapting a certain trial to the needs and preferences of patients in a certain region or population. This ultimately makes Patient Feasibility an essential process for ensuring the trial meets diversity and inclusion criteria.
Country and site selection for clinical trials
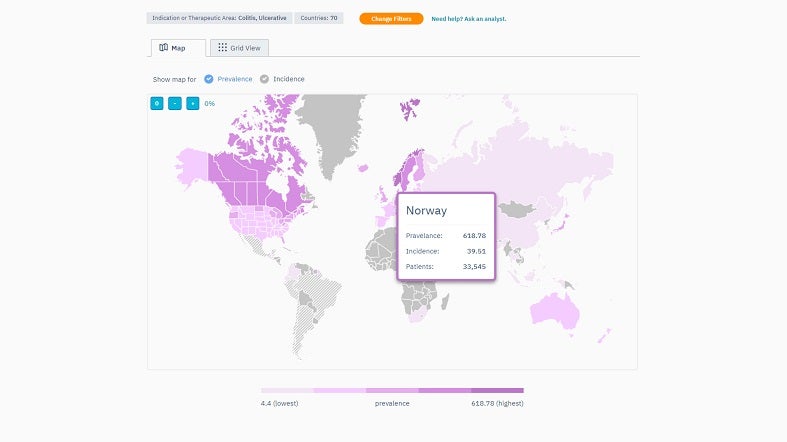
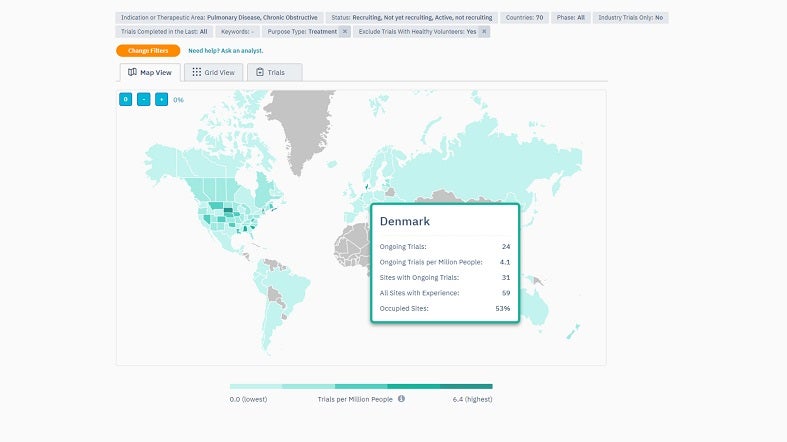
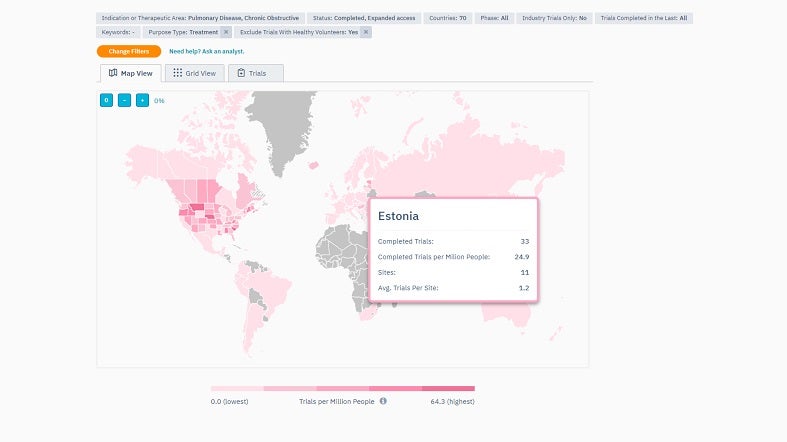
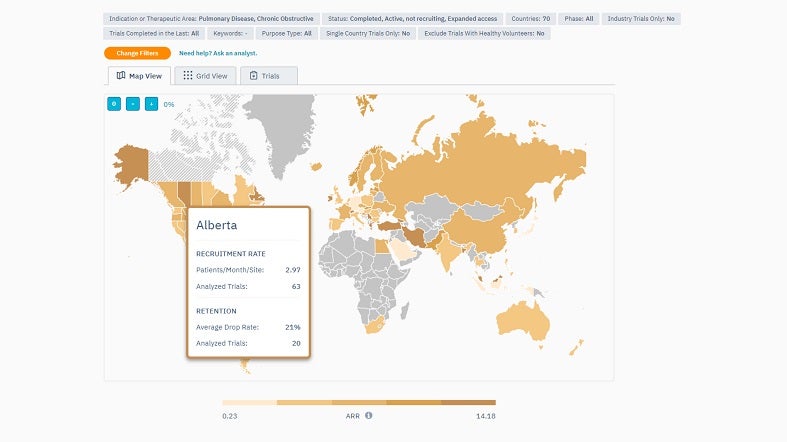
TrialHub’s Feasibility Index is a tool designed to optimise country selection by understanding the local landscape. It provides a map overview, enabling quick country comparisons using criteria such as epidemiology, competition, average recruitment rates, past experiences, and start-up timelines.
By offering a comprehensive view, the Feasibility Index prioritises target countries based on their potential. Users can also pinpoint locations where patients are most aligned with their trials by juxtaposing the trial protocols against local standard-of-care treatments.
To further assist users with site selection, TrialHub connects them with more than 100 local experts in its network, who can deliver unparalleled insights and remove the need for extra vendors.
Successful trials without delays or additional costs
To help ensure easier clinical trial planning, TrialHub allows users to identify and assess potential clinical trial sites that are most likely to recruit patients. This improves the efficiency and effectiveness of their protocols, reducing costs associated with clinical trials by avoiding costly mistakes, such as recruiting patients who are not eligible for the trial or selecting sites that do not have the necessary experience.
TrialHub enables you to generate unique insights by blending vast amounts of verified data sources with your own company data, putting you ahead of the competition.
Trusted feasibility platform for pharmaceutical industry leaders
TrialHub is a trusted partner of leading pharmaceutical and contract research companies such as Novartis, Medpace, ICON, Syneos, Veristat, Premier Research, ProTrials, Bionical Emas, PRC Clinical, and Avance Clinical. To date, TrialHub has been used for the planning of more than 6,000 trials and is praised for its highly accurate data, intuitive interface and outstanding customer support.
To learn more about TrialHub and its advantages, make an enquiry with us today via the form on this page.