
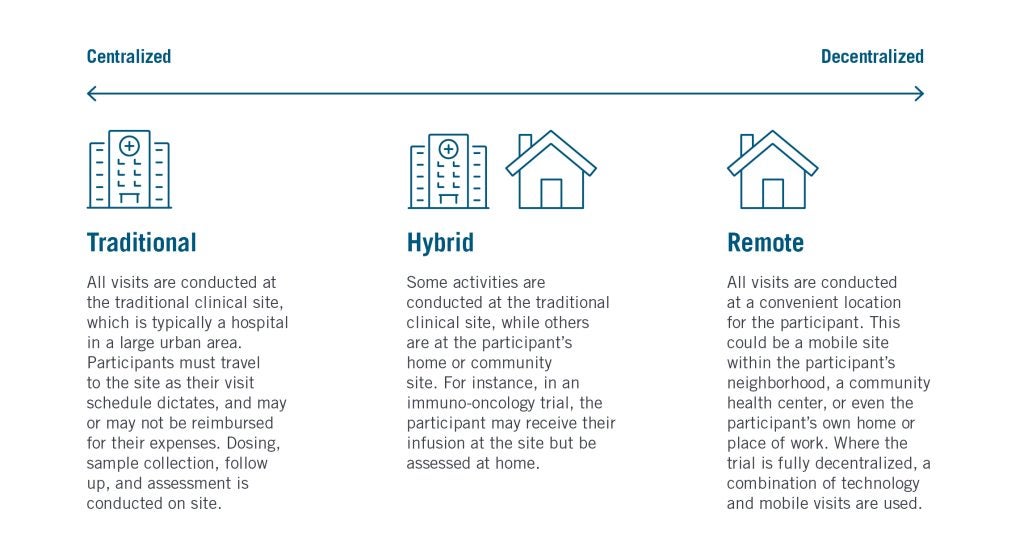
DCTs seek to reduce the number of visits a participant is required to make to a clinical trial site – the traditional location where consent forms are signed, treatments dosed, samples collected, assessments performed, and support provided. With many participants traveling considerable distances to their nearest sites, which are often located in large urban areas, DCTs aim to bring these activities closer to participants’ homes through a multifaceted strategy commonly referred to as community research.
Many DCTs now follow a hybridized design deploying a mixture of conventional site visits and community research methodologies.
In the first seven months of 2024, 1,170 studies included one or more elements of decentralization in their trial protocol, according to GlobalData’s Clinical Trials database. This was 26% higher than the figure for the first seven months of 2023, representing the growing popularity of the DCT model. Below, we break down three key approaches to community research – mobile visits, community sites, and technology – to discuss the benefits of each.
1. Mobile nurse visits
Mobile visits bring aspects of the clinical trial directly to participants, with nurses visiting the participant at their home or a location of their choosing, such as their workplace, school, or hotel. Mobile research nurses align with investigators and site coordinators to deliver in-window visits wherever and whenever works for the participant.
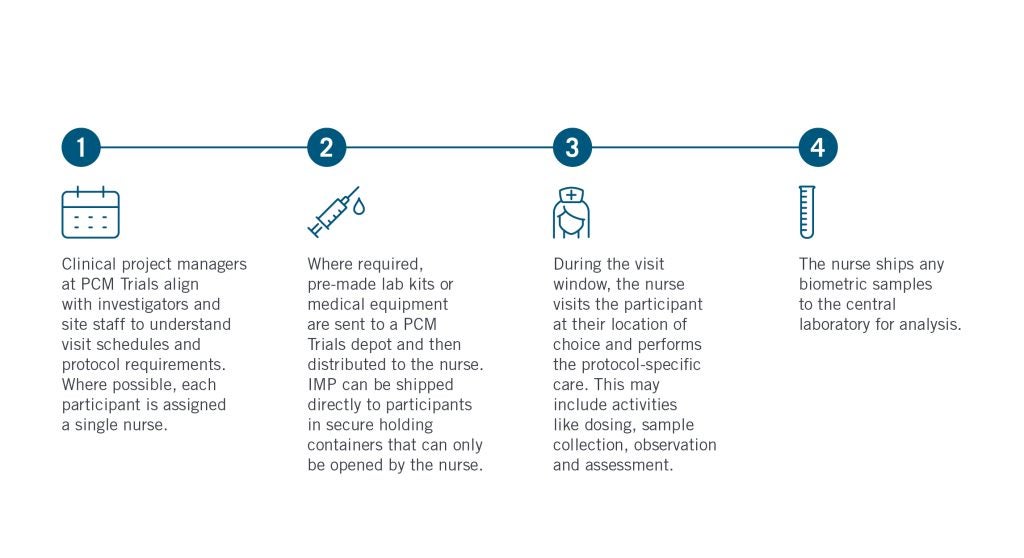
This is often the most convenient option for rare disease patients, children, or those with mobility constraints, thus driving better participation, engagement, and retention for difficult-to-reach participant groups. In the infographic below, we highlight how PCM Trials (the industry’s largest independent DCT site network) sets up a mobile visit for success.
2. Community sites
Community research sites help to expand the reach of the traditional site network ecosystem, establishing sites across various communities to provide convenient access for participants. This can be in the form of mobile research sites, such as those operated by EmVenio Research, a PCM Trials company, which offers a wide network of clinical sites focused on underrepresented communities. EmVenio’s sites include established community research sites and research centers, some of which are exclusively dedicated to a single sponsor or CRO’s study. While these units are fully mobile, with the flexibility to travel between different locations if needed, EmVenio enters communities with long-term placement in mind, taking care to select locations that address clinical trial access disparities for diverse groups of participants while helping to satisfy overall enrollment targets.
Community sites can also be established within existing brick-and-mortar healthcare infrastructure such as health centers, hospitals, and retail pharmacies. For example, in 2024, EmVenio announced a partnership with award-winning national health system Prime Healthcare, allowing the company to conduct research at Prime Healthcare hospitals across the United States and offering Prime’s patients improved access to clinical trials.
3. Technology
Technology has become a key vehicle of decentralization. Where in-person consultations are not necessary, telehealth appointments keep the participant in contact with the study team, enabling frequent yet convenient check-ins. In addition, a range of technologies can be used to collect data directly from participants, whether that involves digital questionnaires and eDiaries recorded in an electronic patient reported outcomes (ePRO) system or the transmission of physiological data from wearables devices directly to the study team.
This use of technology has transformed the scope, depth, and quality of the data that can be collected in a clinical trial, reducing the risk of response bias in questionnaires caused by the potential emotional stress of a hospital visit and enabling the collection of more robust, real-world data.
The benefits of community research
As with the choice between a fully centralized or decentralized study model, choosing between community research strategies is not an all-or-nothing process. Mobile visits or community sites may be necessary for treatment dosing, for example, while remote patient monitoring technology may better support long-term follow-up. Arguably, the most patient-centric DCT is the one which provides a range of options to participants, allowing them to partake in a trial in the way they feel most comfortable.
By designing trials in such a way as to minimize the negative effects on participants, pharmaceutical sponsors could benefit from improved participation and retention figures. GlobalData research has highlighted 50% reductions in dropout rates for Phase III trials with mobile visits compared to similar trials without. In addition, data suggests studies with mobile visits are 41% more likely to complete ahead of schedule than traditional yet otherwise similar trials.
By removing the barriers to access, sponsors not only gather more data but, crucially, more diverse data. Our research reveals studies with mobile visits have been more inclusive of certain historically underrepresented racial and ethnic groups. For instance, while just under 60% of non-mobile visit studies included people of Hispanic/Latino ethnicity, this was 90% for similar studies with mobile visits. Likewise, while just 14% of trials included Native American/Alaskan Native participants, this was 30% in the mobile visit studies.
By extending the reach of the traditional trial network in a hybrid trial, or even going fully remote where appropriate, community research could hold the key to more efficient clinical trials. Check out the following articles to learn more about how DCTs address the problem of healthcare deserts and enable rare disease, pediatric, and geriatric trials, or download the whitepaper below to discover the secrets to successful implementation of mobile visits.


