
Early adoption of artificial intelligence (AI) is already changing the game in clinical trials, with AI enabling some companies to expand their global clinical trial reach by automating the participant engagement process and streamlining data capture. However, global implementation necessitates careful consideration of local regulations, cultures, and the maturity of the healthcare ecosystem.
While AI offers flexible and tailored options for trial participation in a hybrid trial, with potential to make participants feel more connected and supported, it is also important to consider whether its use can create barriers, leaving some participants feeling isolated with unfamiliar technology.
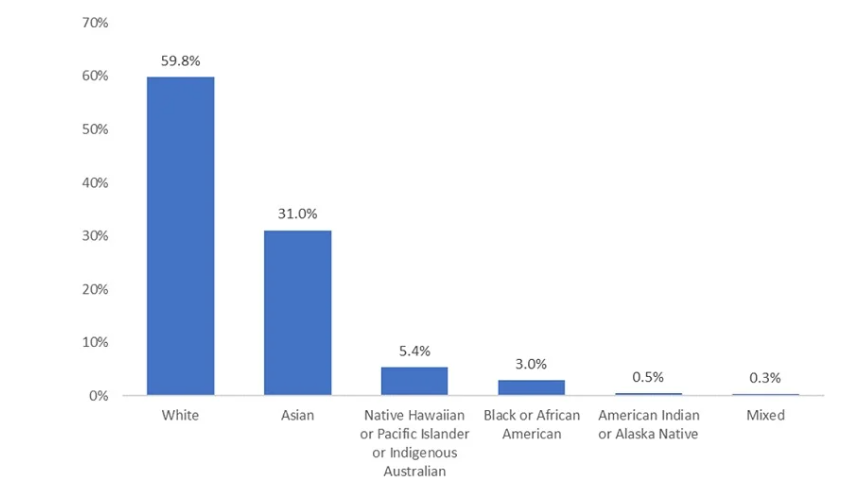
Current diversity challenges in clinical trials
According to data collated by analytics firm GlobalData, recruitment of participants for clinical trials remains a critical challenge, with around 80% of global studies failing to recruit and retain enough participants to enroll on time[i]. The challenge is particularly severe in diverse populations, and the latest FDA report highlights the importance of addressing this. Meanwhile, in the UK, the Health Research Authority (HRA) and Medicines and Healthcare products Regulatory Agency (MHRA) are working to help researchers improve the diversity of participants in clinical research. The aim is to ensure studies involve diverse groups and so enhance understanding of effective treatments and care, thereby reducing health inequalities and ensuring optimal healthcare for all individuals.
AI is set to play a crucial role in expanding inclusivity and efficiency in clinical trials, automating administrative processes that have historically been a bottleneck and rapidly identifying and connecting with underrepresented communities. By reducing the time and effort traditionally required for these processes, AI tools are predicted to significantly increase the efficiency of reaching and engaging diverse populations.
In addition, AI can help accelerate trial recruitment by identifying patient subgroups, simplifying entry criteria, and providing insights into participant behavior. It can even analyze a person’s genetic makeup, physiological data, and lifestyle factors to identify specific patient populations likely to benefit from a drug.
Dr. Amber Hill is founder and CEO of Research Grid, a pioneering AI tech company that has developed an automation engine for admin-free clinical trials. Tools such as those used by Dr. Hill and Research Grid have significantly increased the efficiency of enrolling patients from diverse backgrounds, as shown by the company’s partnership with EmVenio Research, a PCM Trials company providing community research sites.
“What we found is that we can increase engagement by 145%. We can increase efficiency as well by 98% and by reallocating these resources we can decrease costs by anywhere between 45% to 60%,” says Dr. Hill, speaking on a panel discussion in June on How Mobile Visits, Community Sites and AI Support Diversity Goals in Clinical Trials.
“AI is great at administrative processes, predictive analysis and data, outsourcing, understanding where patterns are and where you’re missing gaps in communities that need resourcing in certain areas. [AI] can really dig into the therapeutic area, location, the demographic that you want and really narrow down to the specific communities. Our system automates inclusive engagement, data capture around that engagement, and administration behind that engagement, so that we can reduce bottlenecks.”
Results are impressive, says Dr. Hill: “We’ve increased the patient reach by three times for EmVenio Research and globalized it. The initial reach was 13,000 [participants]; it’s now over 100,000.”
Understanding the challenges
With AI fast becoming an integral part of future studies, the U.S. Food and Drug Administration (FDA) 2024 guidance on AI highlights the need for collaboration, innovation, and harmonization of standards in the use of AI in clinical trials. Focusing on how AI can be used to support clinical trials, a regulatory official speaking at the SCOPE Summit said in February that it will “take time to get there.”
One challenge concerns the potential for biases in generative AI – which refers to algorithms that can produce new content such as codes, text, images and simulations. This could pose a threat to the integrity of clinical trials, with the lack of diversity in trial participants possibly introducing biases in understanding drug effects. To deploy AI effectively, good algorithmic training is needed to avoid such pitfalls. Implementing data safety monitoring boards and mandating the publication of all clinical trial results would create more equitable and unbiased outcomes.
The confluence of AI and community-based research
Using AI in clinical trials brings both benefits and challenges, and its emergence alongside the growing momentum of community-based research adds further potential. While the adoption of community-based clinical research has been a journey over the last twenty years or so, catalyzing events like the pandemic has meant that over the last three years, change has been much faster.
However, this frenetic pace could mean that some sponsors may not have applied the diligence they would traditionally do when considering strategies for deployment, warns Craig Lipset, advisor and founder of Clinical Innovation Partners, at a panel discussion on The Impact of AI on Mobile Visits in April.
“The pandemic was certainly a catalyzing event for the adoption of decentralized trials,” says Lipset. “So how do we make sure that we seize the momentum of a catalyzing event while also making sure that we pace ourselves, that we’re ensuring and sustaining responsible, long-term progress and adoption?”
In a clinical trial featuring elements of decentralization, AI can be used to enhance the participant experience by performing remote health assessments through wearables, heart monitors, and body sensors, reducing reliance on site visits and overcoming accessibility barriers for underrepresented populations, thereby increasing diverse participant access.
Nevertheless, it is important to note that technology-heavy decentralization approaches can be deployed in ways that may not necessarily be patient-centric. Lipset says that ultimately, it will come down to this: “Are we creating space and distance and leaving patients feeling alone and isolated with unfamiliar technology when we’re decentralizing? Or are we using these tools in ways that make a person feel even more connected, even more supported with the research?”
Further, the implementation of community-based research models and the use of AI requires commitment and staying power to succeed. “Eventually the tourists leave and the committed remain – to have conversations about these areas [and] to make sure that we can deliver this sustaining model that creates positive change with the KPIs and the evidence to back it up,” says Lipset.
Putting participants first
AI is fast becoming an integral part of community-based clinical research, with the potential to improve various aspects of the clinical trial process. While the FDA’s 2024 guidance on AI highlights the need for collaboration, innovation and harmonization of standards, experts caution that the use of technology must be measured and remain patient centric.
PCM Trials is a US-based leader in providing mobile research nurses for community-based clinical research, and over the last 16 years has been at the forefront of implementing new technologies into home visit programs. Ellen Weiss, Strategy Decentralized Trials, Emeritus, PCM Trials, says that in terms of decentralization and hybridization of trials, the evolution of the industry is still focused on the importance of patient safety and data: “The more things change, the more they stay the same! We’re maturing, but the fundamentals remain of getting it right and getting the service needed to the patient, where the patient is willing and able to accept it.”
Michelle Kelly, Senior Director Research Technology, PCM Trials, says this new era of AI enablement may present challenges for implementation and adoption, but lessons learned from the past will help to move the industry forward.
“Understanding technology, its pros and cons, and implementing standards harmonization are essential for confidence in deployment,” Kelly says. “I think we are in a unique time where technological advancement is so fast that we need to be more adaptive than we’ve ever been, but at the same time ensuring that we don’t eclipse the individual – the participant who is experiencing the trial on the ground. It’s going to be a very interesting journey over the next few years.”
[i] GlobalData: Thematic Intelligence: Artificial Intelligence in Healthcare, July 2023 https://pharma1.globaldata.com/Analysis/details/Thematic-Intelligence–Artificial-Intelligence-in-Healthcare–2023–74498


