
Mobile research models are making a positive difference in clinical research, helping drug sponsors bring studies to patient populations that have been historically difficult to recruit and retain under the traditional site-based visit model. By conducting trial visits in participants’ everyday environments, patients report an improved trial experience, which can lead to better results for sponsors, including more diverse safety data, reduced burden on sites, and improved participant retention.
In 2024, GlobalData conducted a survey with PCM Trials – the largest independent provider of local clinical trial access globally – to further understand the potential of mobile visits in improving research outcomes. Surveying 100 industry professionals with experience or knowledge of mobile visits, we sought to understand how clinical trial experts perceive the advantages and challenges of implementing mobile nurse visits into studies. The first part in the series revealed the perceived benefits of mobile visits, their effectiveness in meeting study goals, and the types of patient populations they best serve. In this article, we build on this data to envision the future of mobile research services and the challenges and barriers that must be overcome in order to realize their full potential.
A strong future for mobile visits
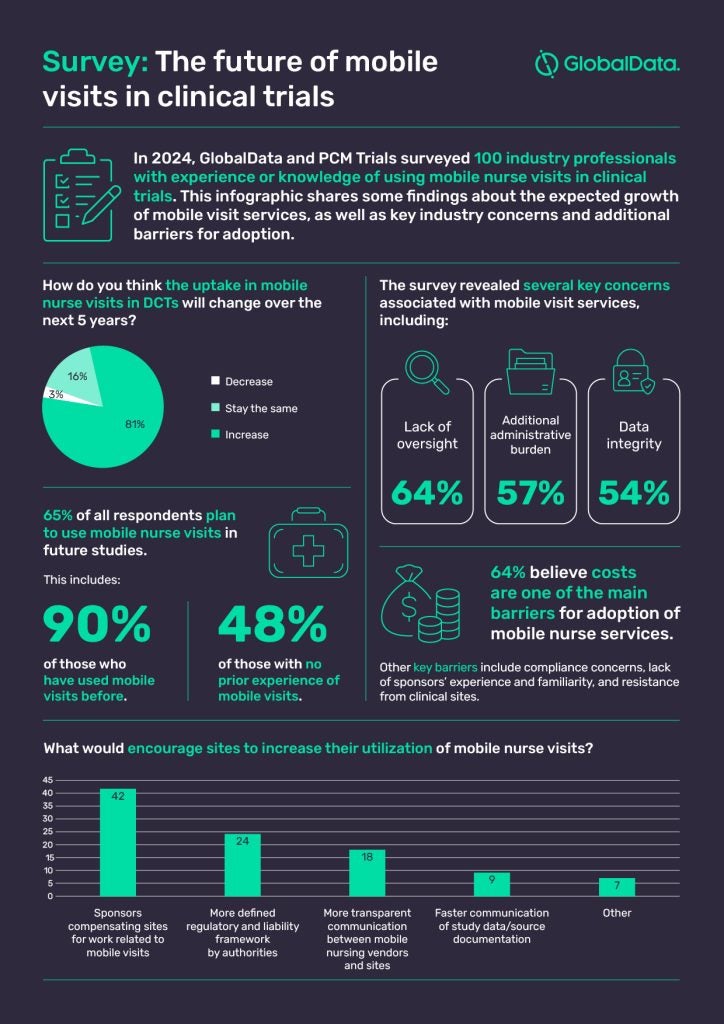
Despite the challenges evident in the infographic above, the survey results paint a promising future for mobile nurse visits. With 81% of respondents expecting the utilization of these services to increase in the near-term future, and only 3% predicting a decline, this alternative way of reaching patients is clearly here to stay.
In addition, almost two-thirds of the respondents plan to use mobile nurse visits in their future studies, including almost half (48%) of those who had no prior experience of doing so. This sentiment was especially strong among those who had worked on previous studies with mobile nurses, with 90% of those individuals stating they plan to use this service again in future studies.
This aligns with broader data on the adoption of decentralized clinical trial (DCT) elements, which incorporates activities such as mobile visits with the use of technology, including telemedicine-based approaches. According to GlobalData’s Clinical Trials database, 2024 was a strong year for DCTs, with 7.44% of clinical trials using one or more elements of decentralization compared to 6.58% in 2023. However, the survey also highlighted several concerns surrounding mobile research, from lack of oversight to additional administrative labor and data integrity risks.
Addressing the challenges
The biggest concern was a potential lack of oversight, since implementing mobile visits requires partnerships with specialist providers or CROs, who must work collaboratively with site staff to ensure strict adherence to study protocols. The criticality of this issue led 64% of respondents to select lack of oversight as a major concern, stressing the importance of partnering with experienced and trusted mobile research vendors with a strong track record of compliance.
Another significant concern from the survey involved a perceived increase in administrative burden, with 57% of respondents selecting this option. There are many logistical considerations involved when implementing mobile visits, including arranging in-window visits with participants, managing mobile clinician schedules, and procuring necessary supplies for visits. PCM Trials has been pioneering the delivery of compliant mobile visits since 2008, and its experienced clinical operations team have played a key role in this success. The team works closely with sponsors and sites through the entire study process, including coordinating visit schedules, ensuring project deadlines are met on time, and providing regular check-ins to drive continual improvement. The company’s nurses are classed as certified mobile research nurses (CMRNs) on account of rigorous onboarding and quality management processes, whereby regular Good Clinical Practice (GCP) and protocol-specific training are required. PCM Trials’ CMRNs are directly hired, trained, certified, and managed by the company, which helps to ensure the quality and consistency necessary for conducting successful clinical trials.
Barriers for adoption
Survey respondents were also asked to identify barriers to further adoption of mobile nurse visits, prompting 64% to choose costs. Other key barriers included compliance challenges and a lack of familiarity and experience amongst sponsors. While upfront costs may be significant, there are multiple possibilities to generate returns over the course of a trial. This is because the use of mobile visits has been shown to help boost recruitment and improve retention of participants, reducing the costs associated with patient dropout and re-enrollment.
Meanwhile, 59% identified resistance from sites as a barrier to future adoption. Much of this stems from revenue loss concerns, since sites are typically paid per each study visit they conduct. If an increasing number of visits are handled remotely and by an external team, the associated payment is not received by the site. However, mobile research still presents opportunities for sites to boost their overall revenue due to its potential to widen the research center’s net by bringing in participants who may not otherwise have enrolled in the trial, like those in underrepresented or rural communities. In a hybrid model, participants may still be required to visit the site for certain parts of the study protocol and may also be supported through telemedicine appointments, for which sites may be compensated.
Nevertheless, a lack of understanding around these opportunities remains a hurdle for the next phase of hybrid trials – and unsurprisingly, when asked how sites could be encouraged to increase their utilization of mobile nurse visits, 42% of respondents said compensation. Other key measures from the survey included more defined regulatory and liability frameworks from regulatory authorities, and better communication between mobile research vendors and sites.
Overall, mobile visits facilitate easier study participation for diverse patient groups by expanding trial access, thereby improving the overall patient experience and enhancing the quality of data collected. However, as challenges and misconceptions with aspects of the DCT model persist, particularly related to cost, logistics, and the role of trial sites, a better way forward for clinical trials depends on the industry’s ability to work through these concerns in partnership with experienced mobile research providers.
To discover more findings from this survey, please click here. Alternatively, you can explore the possibilities of mobile and community-based research in more detail by downloading the whitepaper below.


