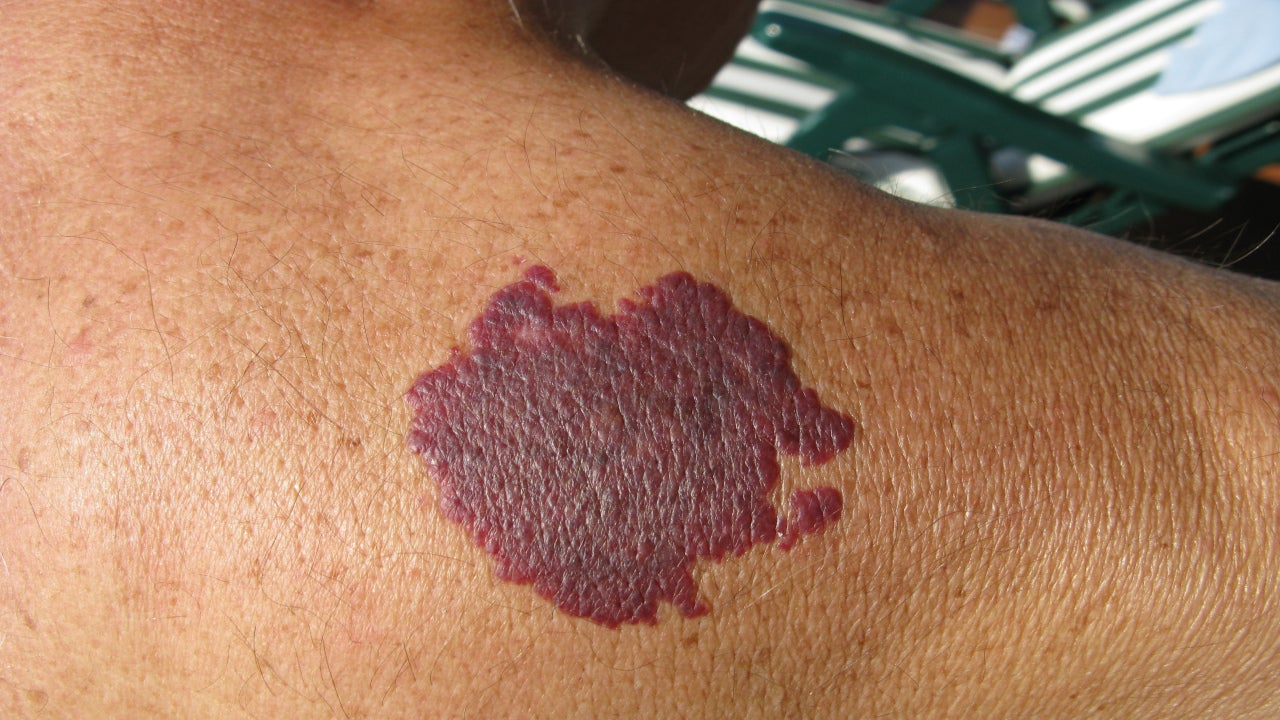
As of 15 February, the likelihood of approval (LoA) for Pfizer’s Rapamune (sirolimus) for Sturge-Weber Syndrome (SWS) rose 15 points, according to GlobalData’s LoA data. The score was based on published results from an investigator-sponsored Phase II/III trial that saw sirolimus achieve a statistically significant improvement in one aspect of the primary endpoint.
The 10-patient Phase II/III) used change in cognitive functioning after six months as its primary outcome. The results were assessed using a panel of testing developed by SWS experts that included measures from the National Institute of Health Toolbox, according to ClinicalTrials.gov. The published data reported a statistically significant improvement in the Pattern Comparison Processing Speed Test, but did not show statistical significance in any other assessments in the primary endpoint, including in measures of executive function, attention, episodic memory, language skills, working memory, or patient-reported emotional functioning (Sebold A.J., et al. (2020) Paediatric Neurology 115: 29-40). Of the ten patients enrolled, one had a moderate upper respiratory infection possibly related to the drug while all other reported adverse events were mild.
While the LoA prior to this news was 8%, GlobalData’s analysis using a combination of machine learning and a proprietary algorithm has raised the LoA to 23%. Rapamune is currently marketed for kidney transplant rejection, angiofibroma and lymphangioleiomyomatosis.
William Newton is a Reporter for Clinical Trials Arena parent company GlobalData’s investigative journalism team. A version of this article originally appeared on the Insights module of GlobalData’s Pharmaceutical Intelligence Center. To access more articles like this, visit GlobalData.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData