Effective Patient Recruitment in Cardiovascular HFpEP Trial
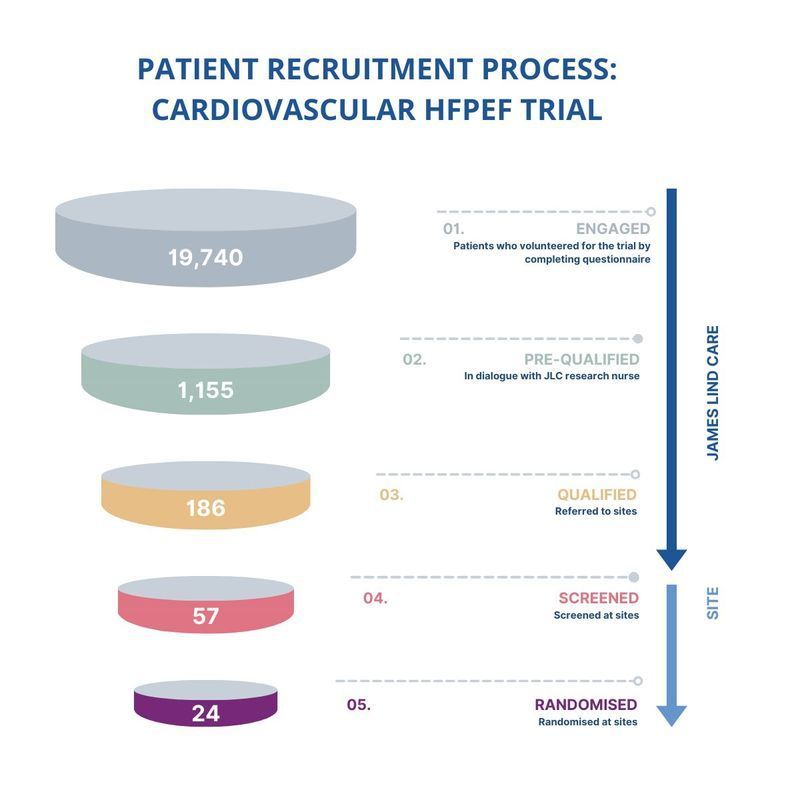
Our success in recruiting patients for the cardiovascular HFpEF trial is a testament to the strength of our patient recruitment process. By taking a patient-first approach and executing a well-organised strategy, we supported two countries, 12 sites, and successfully randomised 24 patients for the trial.
Here’s how we achieved it:
Step 1: Engaging Patients Through Symptom-Focused Communication
We shifted our focus from diagnosis to symptoms of the disease. This approach resonated with patients who may not have been familiar with HFpEF, but were interested in the symptoms. As a result, our patient-targeted communication strategy had nearly 2,000 patients complete the pre-qualification questionnaire.
Step 2: Expert Qualification Process
Our Research Nurse team evaluated each patient’s medical history against the trial’s criteria as well had a phone conversation with the patients to ensure they are fully informed and motivated to participate. Through this process, they identified 186 qualified patients who were eligible and motivated to participate in the clinical trial.
Step 3: Seamless Referral Process and Strong Collaboration
The final step of the recruitment process involved guiding the patients to the trial sites. Our Research Nurses took the lead in providing support throughout to ensure a smooth transition to the sites. This process was enhanced by the collaboration between our Project Team, the sites, and the sponsor, which resulted in a 13% randomisation rate and the successful enrolment of 24 patients.
By focusing on strategic process, we were able to achieve a high randomisation rate and meet the recruitment targets for this cardiovascular trial.