Bevyxxa (betrixaban) for the Treatment of Venous Thromboembolism (VTE) in Adults
Bevyxxa (betrixaban) is a drug developed by Portola Pharmaceuticals that is indicated for the prevention and treatment of venous thromboembolism (VTE) in adults.

You have successfully submitted your enquiry. Someone from our company will respond ASAP
Established in 2004, Tabriz Consulting are pharmaceutical QA consultants serving companies within the pharmaceutical and biotechnology supply chains, from suppliers of APIs, excipients and packaging materials, to primary and secondary manufacturers and those storing or distributing pharmaceutical cold chain and temperate products.
We provide GMP, GDP and validation training, QP services, interim QA management, temperature mapping, QMS development, and GMP and GDP auditing via a team of acknowledged experts in:
Our consultants deliver GMP, GDP and validation training at pharmaceutical QA conferences and seminars in Europe and the USA.
We can also deliver cost-effective on-site GMP, GDP and validation training on a comprehensive range of subject areas. Our highly interactive "Insight" training courses are led by pharmaceutical QA consultants who speak regularly on the international conference circuit. They are, however, tailored to the specific needs of the client and delivered at their own premises.
Our "Insight" client base includes multinational pharmaceutical, biotech manufacturers and pharma-logistics service providers as well as smaller local manufacturers of cold chain packaging materials.
Our consultants are available to provide QP services and QA management on a long-term or short-term contract basis for secondary pharmaceutical manufacturing and APIs. We can provide consultants to support:
Our consultants are currently providing QP services and interim QA management for companies in the UK, the Netherlands and the Republic of Ireland.
In the fiscal year 2005, 43% of all critical and major deficiencies recorded by MHRA’s GDP inspectors were in relation to the control and monitoring of storage and transportation temperatures; temperature mapping is a key tool.
Our temperature mapping service not only accurately tracks how conditions change within a storage area or stability incubator but also includes expert analysis and action planning.
Temperature mapping and reporting gives our customers confidence that their products and / or those of their clients are stored at the specified conditions as well as ensuring that they pass MHRA / IMB / FDA inspections.
Our experienced pharmaceutical QA consultants can help with QMS development by preparing, or advising on the preparation of, key documentation such as technical agreements and standard operating procedures. We can also advise on the design and use of batch record templates.
Whether you have a brand new or an established facility, and whether you require QMS development to support a manufacturer’s or wholesale dealer’s licence, we have the experience to guide you.
One of our most recent projects involved QMS development for a brand-new cold-storage facility and the provision of advice on their application for a wholesale dealer’s licence. The QMS was reviewed and approved by the MHRA during the site inspection.
Tabriz Consulting can provide a comprehensive and professional GMP and GDP audits service to identify areas for improvement. Our pharmaceutical QA consultants can work with your teams to generate realistic improvement plans, or perform due diligence audits on your behalf at potential suppliers and / or contractors. Our consultants are experienced in:
We can also offer GMP and GDP audits to support your site’s preparation for GMP, GDP and PAI inspections by MHRA and EC inspectors and the US F&DA.
Our experienced pharmaceutical QA consultants have conducted pre-FDA audits as well as audits of clinical trials manufacturing facilities, pharma manufacturing facilities, contract laboratories and warehouses in the UK, Europe and Canada.
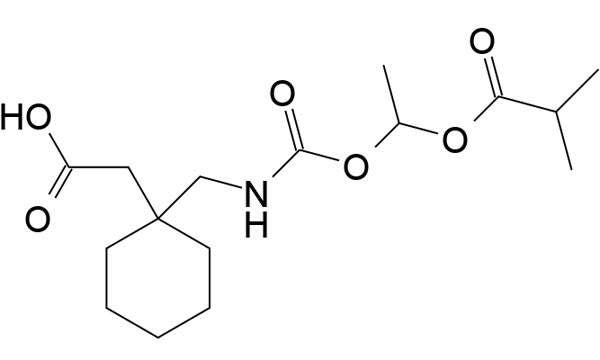
Bevyxxa (betrixaban) is a drug developed by Portola Pharmaceuticals that is indicated for the prevention and treatment of venous thromboembolism (VTE) in adults.
Sublocade™ (buprenorphine extended-release) is a partial agonist indicated for the treatment of moderate-to-severe opioid use disorder (OUD) in adults.