Steglatro (ertugliflozin) for the Treatment of Type 2 Diabetes
Steglatro™ (ertugliflozin) is a combination drug indicated for the improvement of glycaemic control in adult patients with Type 2 diabetes mellitus.

You have successfully submitted your enquiry. Someone from our company will respond ASAP
Wellspring Clinical Services is a leading niche clinical services provider, backed with over 100 years’ experience in pharmaceutical logistics from parent company Mawdsleys.
Wellspring have worked hard to build strong relationships with drug manufacturers and develop global distribution networks, while adhering to strict MHRA guidelines on GDP. Add an unrivalled combination of clinical trial specialists, an in-house procurement team fluent in six languages, and an on-site team of global regulatory experts, and they are the ideal clinical trial partner.
Not only do they have a resident team of EU Qualified Persons, QP (IMPs), and relevant manufacturing and importation authorisations for investigational medicinal products (MIA IMPs), but with their experienced team, Wellspring can design a service to facilitate the smooth distribution of your clinical trial.
In line with GCP and GMP requirements, their experienced clinical labelling specialists can also design, generate and apply all the clinical trial labels you require, in whatever language you need, at a competitive cost and with a rapid turnaround time.
They can source even the most difficult to obtain comparators in single batch, packsize, quantities, or brands in multiple markets. They can provide reassurance on product safety and continuity of supply and take full responsibility for the logistics and QP release as well as guaranteeing complete confidentiality.
Wellspring can also provide you with high-quality placebo supplies and encapsulation services; they can also source all the comparators you need directly from around the globe. So whether you need one hundred placebos or one million comparators, the search ends with Wellspring.
Through parent company Mawdsleys, they can also work in partnership with manufacturers to help make products in clinical trial available on a named patient basis for patients who do not meet the clinical trial protocol. This helps manufacturers create access for their product pre-launch and satisfy advanced demand for the product. It is a key part of a pre-launch program, but it is time-consuming and difficult without a global pharmaceutical logistics network in place. Wellspring can develop a program that is fully compliant to global named patient regulations.
At Wellspring there is a dedicated team of biologists, GMP auditors and QP (IMP)s who can conduct a full audit of your manufacturing premises and, subject to a satisfactory outcome, release your biologic with QP declaration.
Whether your product is an advanced therapeutic product, combined advanced therapeutic product; whether it is a stem cell, tissue or a form of cell therapy, Wellspring has the knowledge and experience to help with QP (IMP) release into Europe.
They can also help with clinical trial applications or legal representation in Europe if needed, as well as creating a patient registry, which is a regulatory requirement in Europe when trialling a biologic, cell therapy, stem cell, advanced therapeutic medicinal product (ATMP), combined advanced medicinal product or medical device.
A team of biological specialists from Wellspring conducted an audit at the premises of Vital Therapies, the developers of a human liver cell-based device. Known as ELAD (extracorpeal liver assist device), the machine acts as an artificial liver taking over a person’s liver function for a few days or weeks to keep them alive until either a transplant becomes available, or the liver regenerates on its own.
The result was that Vital Therapies were then able to navigate the regulations concerning named patient basis and import the advanced therapy product into the UK at the request of a doctor.
Wellspring is currently coordinating the importation, shipping and logistics for the trial, which has already begun in the UK and will be rolled out to Denmark, Spain and France, and will involve over 80 patients.
Steglatro™ (ertugliflozin) is a combination drug indicated for the improvement of glycaemic control in adult patients with Type 2 diabetes mellitus.
Zinbryta (daclizumab) is an injectable formulation jointly developed by Biogen and Abbive for the treatment of relapsing forms of multiple sclerosis (MS) in adults.
Symdeko™ (tezacaftor/ivacaftor and ivacaftor) is a combination drug indicated for the treatment of cystic fibrosis (CF) in people aged 12 years and above.
Lutathera® (lutetium Lu 177 dotatate) is a peptide receptor radionuclide therapy (PRRT) indicated for the treatment of somatostatin receptor positive gastroenteropancreatic neuroendocrine tumours.
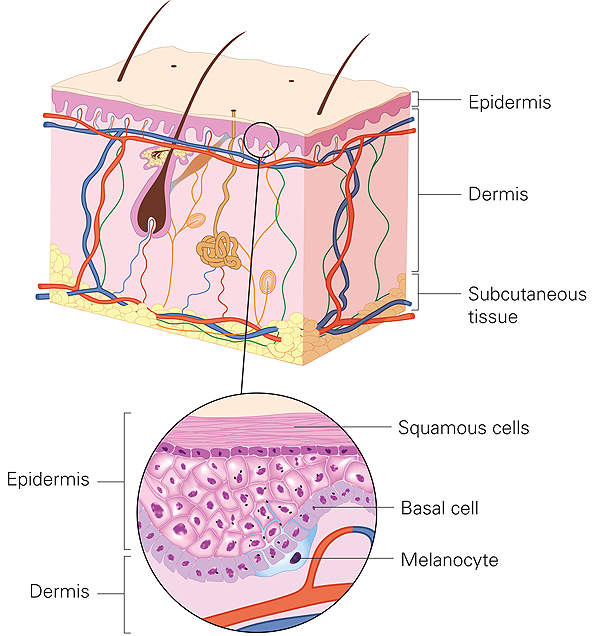
LuxturnaTM (voretigene neparvovec) is approved for the treatment of patients with biallelic RPE65 mutation-associated retinal dystrophy.
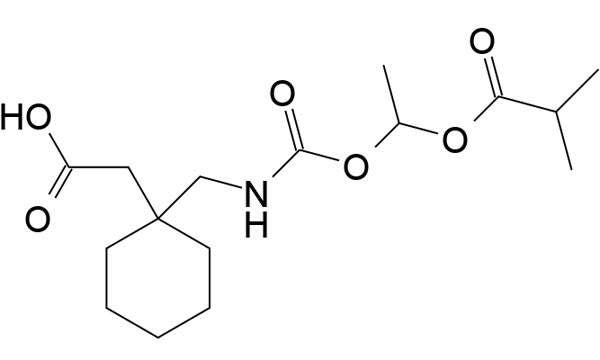
Bevyxxa (betrixaban) is a drug developed by Portola Pharmaceuticals that is indicated for the prevention and treatment of venous thromboembolism (VTE) in adults.

Admelog® (insulin lispro) is a rapid-acting human insulin analogue approved as a follow-on product to treat type 1 and type 2 diabetes in adults and paediatric patients aged three years and older.

Idhifa (enasidenib) is an isocitrate dehydrogenase 2 (IDH2) enzyme inhibitor indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukaemia (AML).
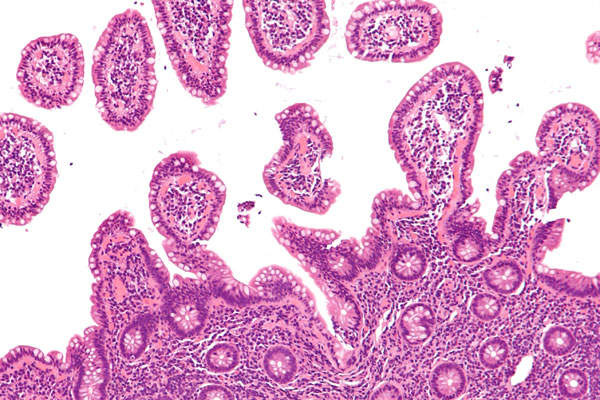
Imfinzi™ (durvalumab) is a monoclonal antibody (mAb) indicated for the treatment of metastatic urothelial carcinoma (mUC).
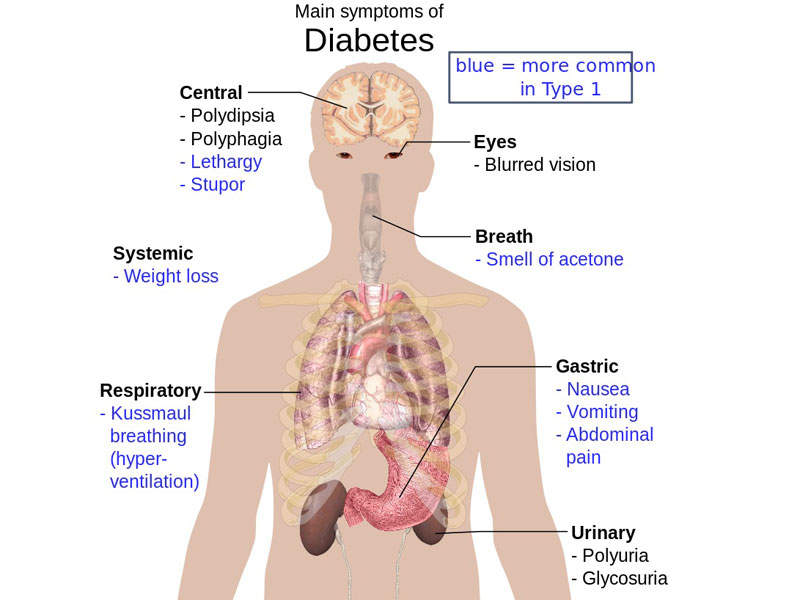
Qtern (dapagliflozin and saxagliptin) is a combination drug with sodium/glucose cotransporter (SGLT) 2 and dipeptidyl peptidase 4 (DPP-4) inhibitors indicated for the treatment of type 2 diabetes.