Kevzara (sarilumab) for the Treatment of Rheumatoid Arthritis
Kevzara® (sarilumab) is a human monoclonal antibody indicated for the treatment of adult patients with moderate-to-severely active rheumatoid arthritis (RA).

Softigel is Procaps' strategic business unit for contract development services. The company supports healthcare and related businesses that develop, manufacture and commercialise pharmaceutical products.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Softigel is Procaps’ strategic business unit for contract development services. The company supports healthcare and related businesses that develop, manufacture, and commercialise pharmaceutical products.
Produced in Colombia, Softgel capsules are have soft gelatin capsules and advanced delivery technologies. They are produced alongside nutritional supplements, veterinary products, and cosmetics.
The company also has facilities in Brazil, Colombia, and Venezuela.
With manufacturing sites in South Africa, Softigel offers various pharmaceutical dosage forms, shapes, and colours, in small to large-scale batches for 38 different countries worldwide.
Softigel focuses on difficult-to-manufacture drugs and aims to capitalise on business opportunities in generic and highly regulated pharmaceutical markets, especially in niche segments with limited competition.
Strong analytical support and expertise in new molecular entities (NME) for highly regulated markets are also available. The company houses a fully integrated product development services (PDS) unit.
Softigel offers access to delivery systems such as novel technology for fixed dose combinations Unigel and Versagels, which provides a new approach to soft capsules without animal derivatives.
Other products include G-Tabs gelatin coated tablets, twist-off capsules, nutritional gummies, omega-3 high-concentrates, tablets, powders, creams, ointments, gels, and hard gel capsules.
The company provides pharmaceutical regulatory support and value-added marketing services. All of its plants fully comply with current good manufacturing practices (cGMP).
The softgel facility is approved by US Food and Drug Administration (FDA) and Medicine and Healthcare Products Regulatory Agency (MHRA). Softigel delivers services to a range of pharmaceutical clients, including Pfizer, Sanofi, Boehringer Ingelheim, and Bayer.
The manufacturing facilities cover product lifecycle phases from early development to commercial and offer single and multiple drug dosage systems.
Procaps’ pilot plant facility for the manufacturing of experimental and pilot batches for softgels, liquids, and tablets uses equipment that replicates lab-scale and small-scale non-good manufacturing practices (GMP) and GMP batches, up to full-scale commercial GMP.
The product development team provides clients with access to formulation and processing clinical trial manufacturing, scale-up, analytical development, and regulatory support for markets including the US, Europe and Latin America.
The company assists clients from Phase I to registration and large-scale commercial production.
Softgel dosage forms have a range of benefits, including:

Kevzara® (sarilumab) is a human monoclonal antibody indicated for the treatment of adult patients with moderate-to-severely active rheumatoid arthritis (RA).
Mavenclad™ (cladribine tablets) is a selective immune reconstitution therapy indicated for the treatment of active relapsing multiple sclerosis.
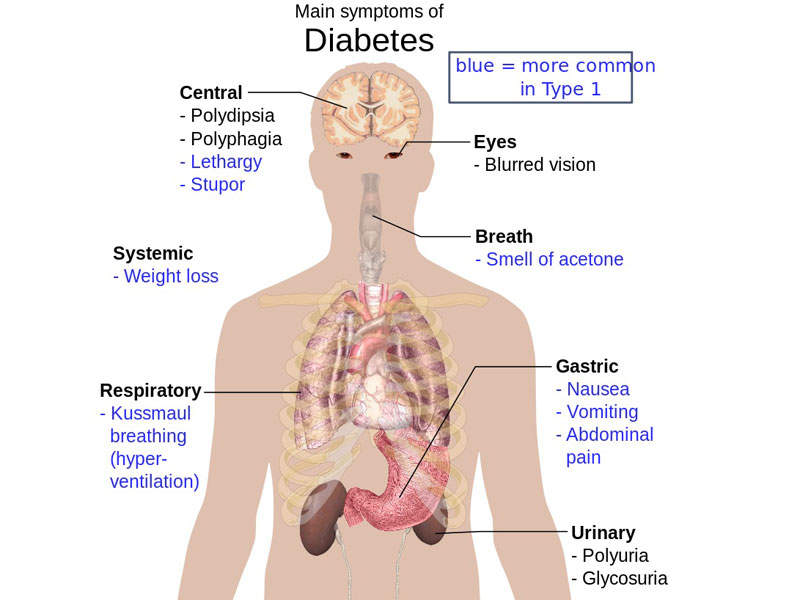
Qtern (dapagliflozin and saxagliptin) is a combination drug with sodium/glucose cotransporter (SGLT) 2 and dipeptidyl peptidase 4 (DPP-4) inhibitors indicated for the treatment of type 2 diabetes.

Eliquis (apixaban) is an anticoagulant drug that was jointly developed by Bristol-Myers Squibb and Pfizer.
Aristada™ (aripiprazole lauroxil), discovered and developed by Alkermes, is an antipsychotic drug indicated for the treatment of schizophrenia in adults.