Understanding Sucrose: Matching Sucrose Quality to Functional Need
Raw source (Cane vs Beet)-Cane derived generally has higher residual b-glucan load than beet derived sucrose, which can carry over into final product.

Pfanstiehl is a manufacturer specialising in scale-up development, custom synthesis, and purification of injectable, high-purity low-endotoxin (HPLE) carbohydrates.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Pfanstiehl is a manufacturer specialising in scale-up development, custom synthesis, and purification of injectable, high-purity low-endotoxin (HPLE) carbohydrates. It also produces highly potent active pharmaceutical ingredients (API), and pharmaceutical intermediates.
The US-based company works with amounts from gram to multi-tonne commercial quantities, serving the pharmaceutical, biotechnology, veterinary, and cosmetics markets with custom carbohydrate synthesis and development. These follow current good manufacturing practices (cGMP) and International Conference on Harmonisation (ICH) guidelines.
Protein stabilisation can be achieved by using Pfanstiehl’s high-purity ingredients (HPI). Trehalose and sucrose can stabilise proteins, lipids, and carbohydrates throughout freeze and formation cycles of remedial treatment. They can also help improve recovery of bioproduction cells and those used in cell therapy.
Applications of this method include for vaccines, stem cell technology, monoclonal antibodies (mAb), and antibody fragments (fAbs). They are also often used in the cryopreservation process. Sucrose is preferred over trehalose in cases where viscosity and solubility are an issue.
In addition, trehalose can be produced for drug delivery, as a stabiliser of vaccines, and as a lyoprotectant.
Manitol is produced by reducing sugar and is often used as a bulking agent in tablets. It can be used to stabilise liquid formulations and vaccines, protecting proteins from reconstitution, denaturation upon lyophilisation, and spray drying. It can also be used as a lycoprotectant. Pfanstiehl’s D-Mannitol is available in packet sizes, including 1kg, 5kg, and 25kg.
Another protein stabilisation product offered by Pfanstiehl is high-quality, pharmaceutical grade maltose. This product can be used in certain countries to stabilise fluids. Other applications include blood fractionation, as a bulking agent, and to prevent protein aggregation in intravenous immunoglobulin (IVIG) solutions.
Pfanstiehl’s HPLE mannose and galactose play key roles in the optimisation of protein therapeutics bioproduction. Mannose is important in the human metabolism and is critical to ensure the potency, consistency, and yield of therapeutic glycosylation. Pfanstiehl specifically develops these carbohydrates for biopharmaceutical use and the high-quality standards of the industry.
Galactose has an added benefit of mitigating lactate and ammonia formation throughout the optimisation process. This monosaccharide sugar is found in beets, mucilages, and gums. It is used as a modulator in protein bioproduction.
Pfanstiehl’s main areas of specialism include carbohydrate chemistry for rare and blocked sugars, advanced intermediates, as well as potent or cytotoxic compounds.
It develops linker-toxins to support ADC manufacture and nucleoside chemistry for generic APIs.
The Special Chemicals Corporation was founded by Carl Pfanstiehl. He began by offering enzymes, high-purity carbohydrates, and amino acids to analytical laboratories and hospitals. By 1930, manufacturer and distributer of dairy supplies Babson Brothers joined, helping with finances and management. The company was renamed Pfanstiehl Chemical Corporation.
The company became a subsidiary of Babson in 1942, operating its dairy sanitary, metallurgical, and fine chemicals divisions. Throughout the late 1940s and into the early 1950s, the company gained new members and began producing biochemical in larger entities. By 1959, the company shifted its focus towards carbohydrates and other organic chemicals.
It was at this point Pfanstiehl significantly expanded its capacity by moving to its current location in Waukegan. Over the next 50 years, the company worked closely with its pharmaceutical and biopharmaceutical clients.
Its current main focus is to synthesise commercial and proprietary compounds. It designs its new facilities, conducts procedures, and trains staff to meet or exceed US Food and Drug Administration (FDA), current good manufacturing practice (cGMP), occupational safety and health administration (OSHA), and international regulatory and multi-compendial standards.

Raw source (Cane vs Beet)-Cane derived generally has higher residual b-glucan load than beet derived sucrose, which can carry over into final product.

In the pharmaceutical industry, products which benefit from the stabilizing effects of trehalose include, but are not limited to monoclonal antibodies (mAbs), antibody drug conjugates (ADCs), fusion proteins, peptides, stem cells and vaccines.
ILUMYA™ (tildrakizumab-asmn) is a subcutaneous infusion indicated for the treatment of adults with moderate-to-severe plaque psoriasis.

After 100 years in the business, Pfanstiehl has launched high purity, low endotoxin, low metal L-Histidine and L-Histidine Hydrochloride Monohydrate, establishing an innovative quality standard for amino acids.

Pfanstiehl has announced that it will be sponsoring the 2019 Colorado Protein Stability Conference.
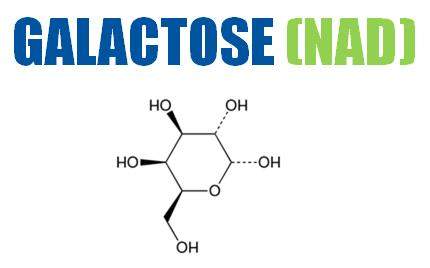
Galactose is a monosaccharide sugar of the hexose class that is a constituent of lactose and many polysaccharides.
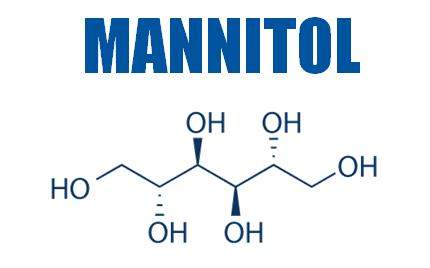
Mannitol is classified as a sugar alcohol and is derived from a sugar (mannose) by reduction. Other widely known polyols are xylitol and sorbitol.
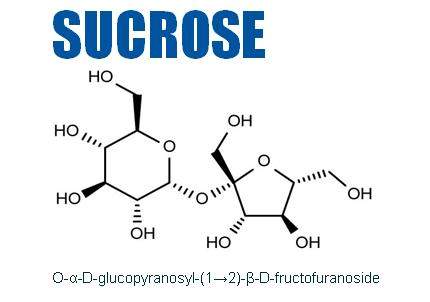
Sucrose is a non-reducing crystalline disaccharide made up of glucose and fructose, found in many plants but extracted as ordinary sugar mainly from sugar cane and sugar beets.
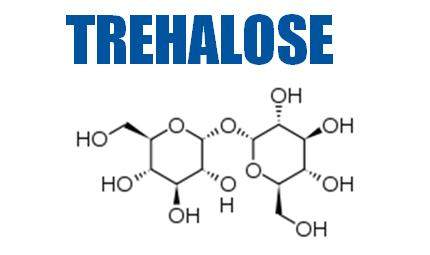
Trehalose is a non-reducing disaccharide consisting of two glucose molecules linked by an α, α–1,1 bond.

Vice-president of research and development (R&D) Dr Trevor Calkins discusses Pfanstiehl’s active pharmaceutical ingredients (API) contract development and manufacturing organisation (CDMO) philosophy and capabilities at the CPhI/InformEx exhibition