Nuplazid (pimavanserin) for the Treatment of Parkinson’s Disease Psychosis (PDP), USA
Nuplazid (pimavanserin) is an atypical antipsychotic drug indicated for the treatment of Parkinson’s disease psychosis (PDP).

You have successfully submitted your enquiry. Someone from our company will respond ASAP
Finkler is an independent consultant company that has been offering first-class regulatory affairs and generic drug development services since 1996.
Led by a team of experienced professionals, Finkler offers customised regulatory affairs and development solutions to ensure fast and successful development, authorisation or maintenance of your products.
Our key services in regulatory affairs are strategic advice, documentation, regulatory project / submission management and lifecycle management / maintenance.
We offer sound strategic advice for regulatory affairs. As part of our service we can work with you to develop suitable development strategies. These include:
Our regulatory affairs documentation services include compilation of dossiers according to Common Technical Document standards (ectd and Nees according to current requirements). Other documentation services include:
We ensure that your applications and dossiers meet the relevant requirements and that the documentation is prepared in a timely manner. We follow up with our customers’ internal departments and their external partners during all phases of the procedure.
We handle your decentralised and mutual recognition procedures as well as your national applications from submission to approval. Our team of experts can prepare answers to deficiency letters. We liaise with all national EU authorities and with the European Medicines Agency (EMA) at all stages of the procedure.
For both variations and renewals state-of-the-art documents are needed to keep your products alive. We ensure that your updates are prepared according to recent EU requirements.
Our skilled team of pharmacists and medical doctors has many years of experience in the development of generic drug products within the EU. We understand that no two development projects are the same and that each customer has its own goals. This is why we tailor our services not only to meet or exceed EU regulatory agencies’ needs but also to meet specific customer requirements.
Our deep knowledge of regulatory affairs ensures that the achievements and results from development work are presented in an agency-friendly way, to ensure fast and successful approval.
We provide customised half-day to three-day seminars at our offices in Lörrach, Germany, or at the customer’s site.
Since regulatory affairs is our daily business the content of the programmes is based on real-world experience combined with theoretical background knowledge. Seminar options include:
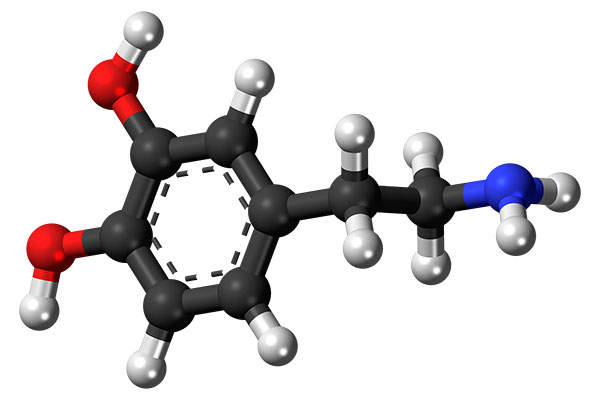
Nuplazid (pimavanserin) is an atypical antipsychotic drug indicated for the treatment of Parkinson’s disease psychosis (PDP).

Ozempic® (semaglutide) is a glucagon-like peptide (GLP-1) receptor agonist indicated for the treatment of Type 2 diabetes in adults.
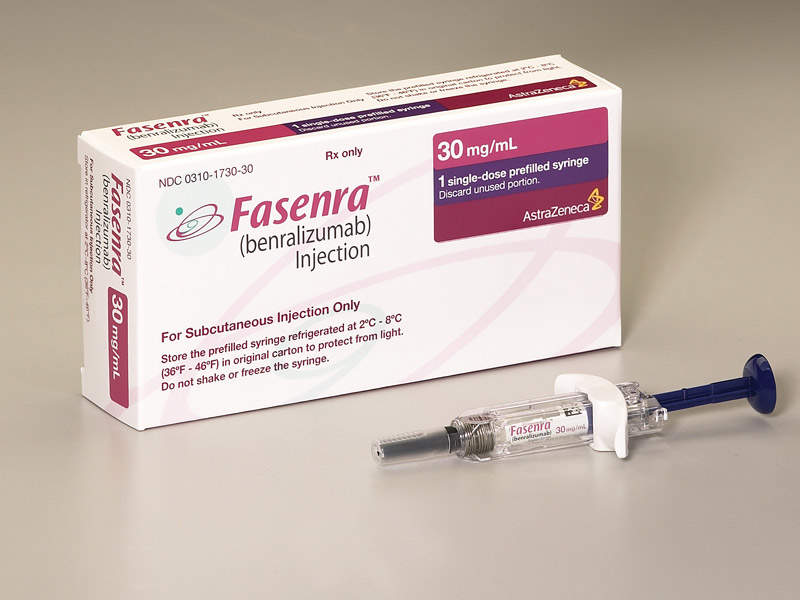
Fasenra (benralizumab) is a monoclonal antibody (mAb) indicated for the treatment of severe eosinophilic asthma in patients aged 12 years and older.
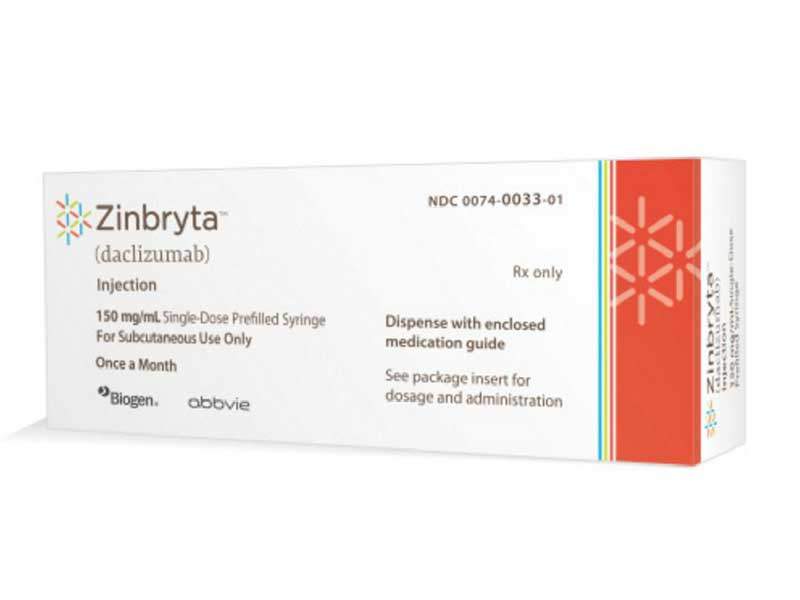
Zinbryta (daclizumab) is an injectable formulation jointly developed by Biogen and Abbive for the treatment of relapsing forms of multiple sclerosis (MS) in adults.
Faslodex (fulvestrant) is an oestrogen receptor antagonist indicated for the treatment of locally advanced or metastatic breast cancer.
Mydayis is a CNS stimulant drug indicated for the treatment of attention deficit hyperactivity disorder (ADHD) in patients aged 13 years and above.
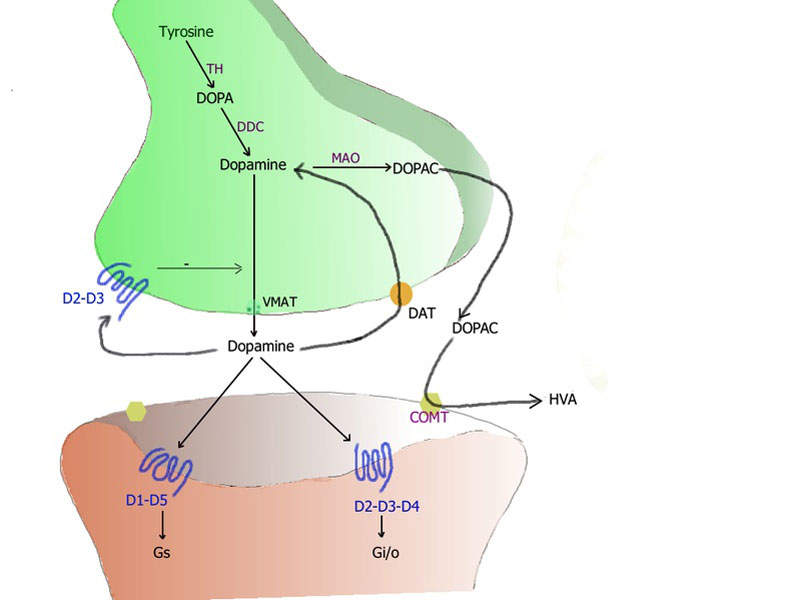
Xadago® (safinamide) is a monoamine oxidase type B (MAO-B) inhibitor indicated for the treatment of Parkinson's disease.
Developed by Sanofi, Soliqua 100/33 (insulin glargine & Lixisenatide injection) is indicated for the treatment of Type 2 diabetes.
Adlyxin (lixisenatide) is an injectable solution developed by Sanofi for the treatment of type 2 diabetes in adults.