Fasenra (benralizumab) for the Treatment of Asthma
Fasenra (benralizumab) is a monoclonal antibody (mAb) indicated for the treatment of severe eosinophilic asthma in patients aged 12 years and older.

McCarthy Consultant Services Inc. (MCSI) provides expert regulatory affairs consulting. As a global industry leader, MCSI understands the importance of sound regulatory management and client satisfaction.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
McCarthy Consultant Services Inc. (MCSI) provides expert regulatory affairs consulting. As a global industry leader, MCSI understands the importance of sound regulatory management and client satisfaction.
Our ability to tackle difficult and complex regulatory issues quickly and efficiently has made MCSI a trusted and valued provider of regulatory affairs services to the biotechnology, pharmaceutical, medical device, natural health product, food and cosmetic industries.
MCSI is considered a major asset by Canadian, foreign and virtual organisations conducting business in Canada, the US and EU. With our regulatory capabilities, we can successfully guide your products through complicated regulations.
MCSI has built worldwide, strategic alliances with organisations, allowing us to offer our clients access to the UK, Australia, Japan, the Nordic countries, Russia and Eastern Europe.
We can provide a range of services, from offering well-established, multinational companies an expert opinion, to outsourcing all regulatory affairs for small startup firms.
Whether it is regulatory strategic planning or a full product submission, MCSI has an expert team of regulatory associates who can get your project completed.
Along with regulatory strategic planning and preparation and filing of product applications for drugs, medical devices, natural health products, MCSI also offers a wide range of other regulatory affairs and assurance services, including:
MCSI has worked on various regulatory and quality assurance projects, including:
To discuss your regulatory outsourcing needs, please get in touch with us using the enquiry form below.
Fasenra (benralizumab) is a monoclonal antibody (mAb) indicated for the treatment of severe eosinophilic asthma in patients aged 12 years and older.
Zinbryta (daclizumab) is an injectable formulation jointly developed by Biogen and Abbive for the treatment of relapsing forms of multiple sclerosis (MS) in adults.
Austedo™ (deutetrabenazine) is a vesicular monoamine transporter 2 (VMAT 2) inhibitor indicated for the treatment of chorea associated with Huntington’s disease.
Alunbrig (brigatinib) is a tyrosine kinase inhibitor indicated for the treatment of patients with anaplastic lymphoma kinase-positive (ALK+) metastatic non-small cell lung cancer (NSCLC).
Dysport (abobotulinumtoxinA) is an injectable formulation developed by Ipsen Biopharmaceuticals for the treatment of lower limb spasticity in paediatric patients aged two years or older.

Xiidra lifitegrast ophthalmic solution is a twice-a-day eye drop solution developed by Shire as a treatment for the signs and symptoms of dry eye disease in adults.
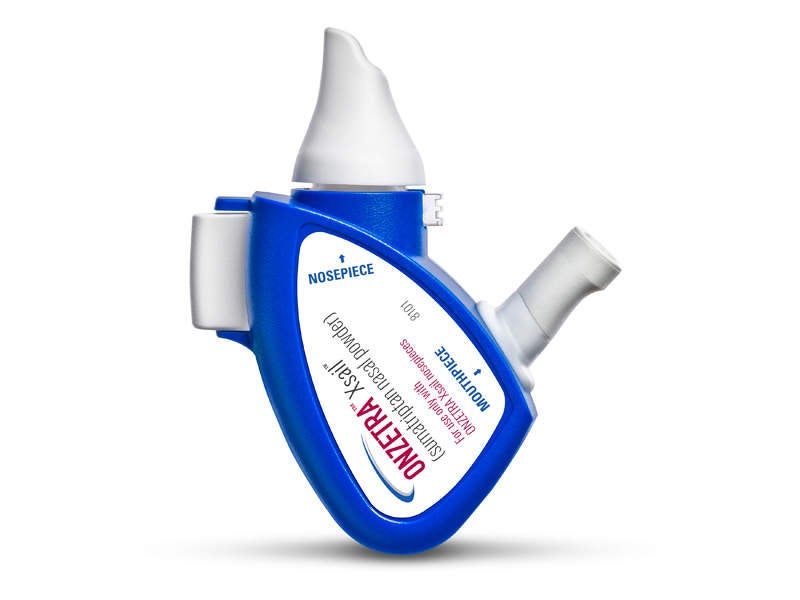
ONZETRA Xsail is a Food and Drug Administration (FDA)-approved intranasal dry powder formulation developed by Avanir Pharmaceuticals.
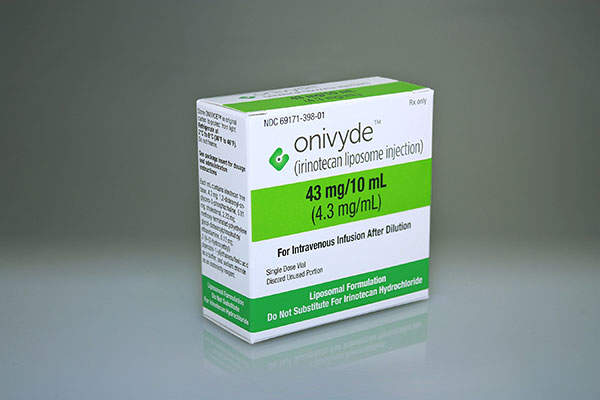
Onivyde (MM-398 / irinotecan liposome injection) is an intravenous injection indicated for the treatment of metastatic pancreatic cancer.