Nuplazid (pimavanserin) for the Treatment of Parkinson’s Disease Psychosis (PDP), USA
Nuplazid (pimavanserin) is an atypical antipsychotic drug indicated for the treatment of Parkinson’s disease psychosis (PDP).

Pharmaceutical Development Group (PDG) provides pharmaceutical regulatory consulting to firms located around the world.
You have successfully submitted your enquiry. Someone from our company will respond ASAP

Pharmaceutical Development Group (PDG) provides pharmaceutical regulatory consulting to firms located around the world.
The company offers a one-stop-shop for US Food and Drug Administration (FDA) new drug application (NDA) compilation and submission, design and execution of toxicology and clinical programmes (Phases I-IV), sourcing and oversight of third party vendors including raw material suppliers, contract laboratories, contract research organisations (CRO), contract manufacturing organisations (CMO), and pre-approval inspections of all facilities.
Having facilitated hundreds of FDA pre-submission meetings and regulatory submissions for its clients, PDG knows how to determine and navigate the best regulatory pathway for your drug development project.
From submitting a Citizen Petition to changing a monograph or submitting a 505(b)(2) to repurpose a discontinued drug efficacy study implementation (DESI) drug, through priority review and accelerated approval of a new chemical entity, PDG has the expertise to see your project through.
While this is never entirely possible, the model characterises the extent PDG prepares its submissions, and clients for FDA meetings.
The company’s approach to determining and illustrating risks and benefits integrates products, patient populations, and disease states with toxicology, pharmacokinetics, pharmacodynamics, clinical, statistical, epidemiology, CMC, labelling, and pharmacovigilance concerns.
PDG facilitates innovation, prolongs product lifecycles, evades generic competition, supplements dwindling pipelines, and differentiates or repurposes existing drug products.
As a regulatory strategist and pharmaceutical consultant, PDG understands that improvements in safety, efficacy, bioavailability, patient compliance, and convenience are viewed favourably by the FDA, as well as the marketplace.
The company knows through first-hand drug development experience how to help you develop novel formulations. It can also help you devise orphan, combination, or reformulation-based product development and other repositioning strategies that have the potential of yielding high returns on investment (ROI).
Typical of our services, but not entirely inclusive are the following:
The optimal time to engage a consultant such as PDG is in advance of meeting with FDA. However, PDG will engage at whatever point we are called upon, including regulatory emergencies such as those caused by unplanned audits or unanticipated inspection results.
PSG is ready to deploy for its clients 24/7 in all time-zones, including travelling to your facility when needed.
Nuplazid (pimavanserin) is an atypical antipsychotic drug indicated for the treatment of Parkinson’s disease psychosis (PDP).

Spritam (levetiracetam) is an adjunctive therapy indicated for the treatment of seizures in patients with epilepsy, the fourth most common neurological disorder affecting people of all age groups.
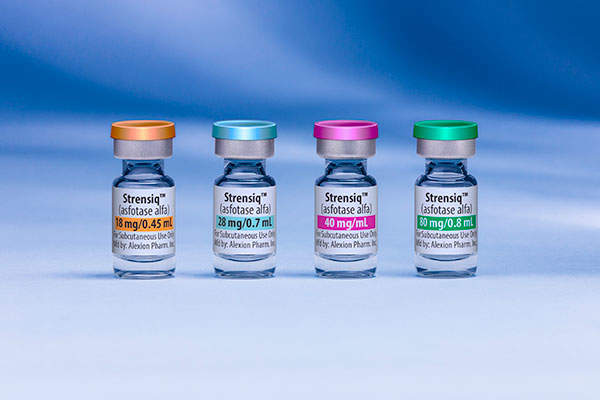
Strensiq (asfotase alfa) is the first enzyme replacement therapy (ERT) approved in the US for the treatment of perinatal / infantile and juvenile-onset hypophosphatasia (HPP), which is a life-threatening and ultra-rare metabolic disorder.

Daklinza (daclatasvir), an NS5A replication complex inhibitor, is the first 12-week, all-oral therapy indicated for use with sofosbuvir, for the treatment of hepatitis C (HCV) genotype 3 infections.
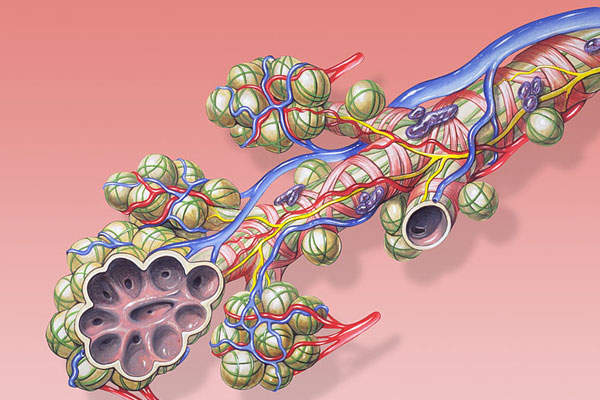
Anoro ellipta (umeclidinium bromide / vilanterol) is a dry powder for inhalation, indicated for the treatment of airflow obstructions in patients suffering from chronic obstructive pulmonary disease (COPD) or emphysema.