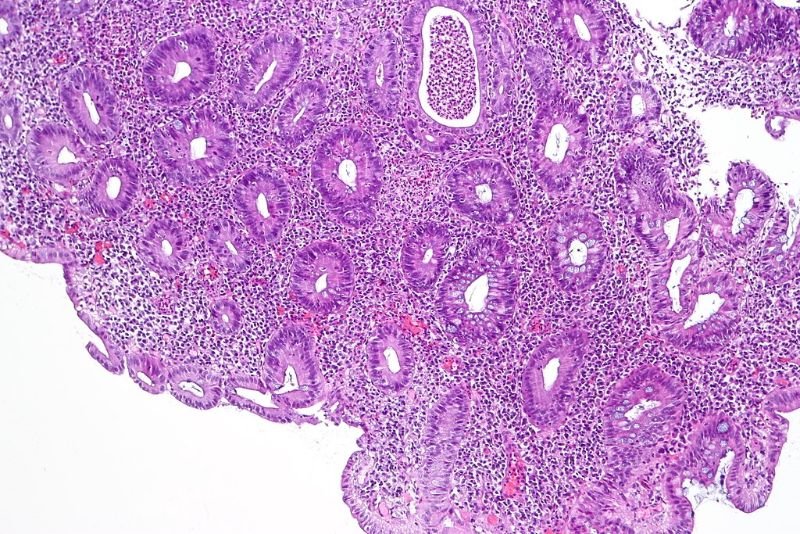
Abivax has received approval from the French National Regulatory Authority (ANSM) to conduct its Phase IIb clinical trial of ABX464 to treat moderate-to-severe active ulcerative colitis (UC) patients in the country.
The trial is designed to be conducted in a total of 232 patients at approximately 150 sites. The ANSM approval brings the total number of countries to 15.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
ABX464 attaches to the cap binding complex (CBC) and boosts its biological functions in biogenesis of cellular RNA. The candidate’s mechanism allows its use as an anti-inflammatory therapy.
Named ABX464-103, the randomised, double-blind, placebo-controlled, dose-ranging Phase IIb is assessing a 25mg/day, 50mg/day and 100mg/day once-daily dose of the drug compared to placebo.
It comprises a 16-week induction phase and an open-label maintenance study. Patient recruitment for the trial was commenced in August this year.
The primary endpoint is a decrease in modified Mayo Score at eight weeks, while secondary endpoints include clinical remission, endoscopic improvement and biomarker faecal calprotectin.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTop-line results from the induction phase are expected to be available by the end of next year.
Abivax CEO Dr Hartmut Ehrlich said: “As presented during UEG week in Barcelona (19-23 October 2019), our proof of concept Phase IIa studies with ABX464 in patients with moderate to severe UC showed that 75% of all evaluable patients were in clinical remission and therefore essentially free of symptoms after two months induction and 12 months maintenance.
“With the ABX464-103 study (Phase IIb), we are planning to confirm these excellent outcomes in a statistically relevant number of patients and, at the same time, evaluate different doses of ABX464 to define the optimal dose for subsequent Phase III testing.”
ABX464 is also being evaluated in the Phase IIa ABX464-301 trial in 60 moderate-to-severe active rheumatoid arthritis patients across France, Poland, Belgium and Hungary.
Top-line data from three-month induction therapy will be reported next year.
The company is planning to launch another Phase IIa trial in the first quarter of next year to assess the drug candidate to treat Crohn’s disease patients.





