A joint venture between DemeRx and Atai Life Sciences has been cleared by the UK Medicines and Healthcare products Regulatory Agency (MHRA) to start enrolment for a Phase I/IIa trial of ibogaine HCl (DMX-1002) in the treatment of opioid use disorder (OUD).
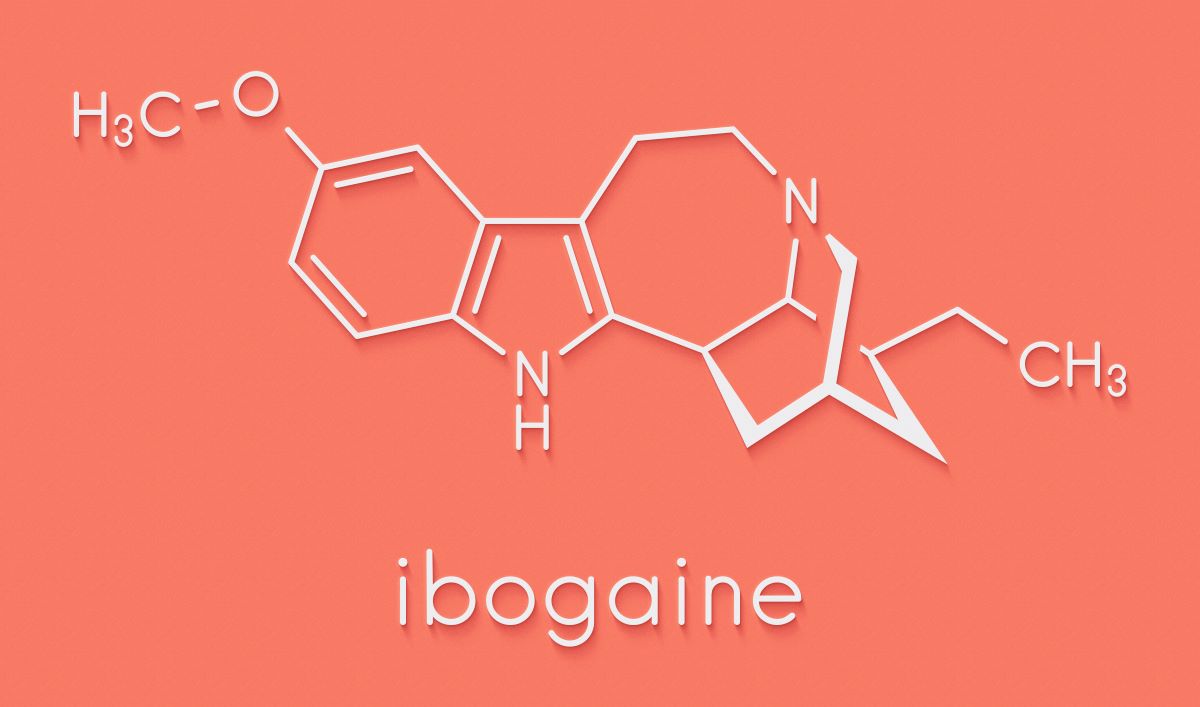
Ibogaine is a naturally occurring psychoactive compound isolated from a West African shrub called iboga. Atai has invested $22m in the joint venture with DemeRx to develop the psychedelic, which has shown evidence of rapid and sustained efficacy in treating opioid use disorder (OUD).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The substance has previously been sold as a stimulant and antidepressant in France and studied elsewhere for addiction treatment, most notably in a large study led by DemeRx CEO Dr Deborah Mash in St. Kitts, West Indies.
“Not only were patients able to safely and successfully transition into sobriety, we found no evidence of additional abuse potential,” said Mash. “Given the limitation in currently available treatments, ibogaine represents an enormous leap forward for OUD sufferers.”
Phase I of the MHRA-approved trial will take place at the Manchester clinical unit of MAC Clinical Research (MAC). Atai and DemeRxsaid MAC’s extensive good clinical practice experience, expertise and infrastructure will provide a platform for what is hoped to be a successful and significant trial.
The approval for a Phase I/IIa clinical trial will allow DemeRx to study ibogaine in recreational drug users before starting a second trial stage in opioid-dependent patients. Prior to starting this stage, the trial will pause to allow MHRA to evaluate human safety data and nonclinical study results.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Patients will be enrolled into the trial through a press release, company websites, social media and local adverts,” Mash told Clinical Trials Arena. “110 patients will be recruited in total, including 30 recreational drug users (healthy volunteers) in Stage 1 and 80 opioid-dependent patients in Stage 2.”
“Our clinical trial authorisation is a critical milestone,” said Mash. “Stage 1 of the study will provide assessment of safety through the evaluation of ibogaine’s potential adverse effects before we move to the proof-of-concept efficacy portion of our study in patients who seek to detoxify from opioids.”
The MHRA regulatory approval comes as a breakthrough for patients looking to escape the consuming and often deadly cycle of opioid dependence.
“Timing could not be more important as the world faces an ever-growing opioid epidemic,” said Atai Life Sciences CEO and co-founder Florian Brand. “Current treatment options are not highly effective; approximately 75% of patients undergoing OUD therapy experience relapse within one year of treatment.”
The opioid crisis highlights a critical unmet medical need, particularly in the United States. In 2018 alone, 2.1 million Americans met the diagnostic criteria for OUD, while 47,600 people died from opioid overdoses.
People seeking to overcome opioid addiction are often impeded in their recovery by the current treatments available. Even if they do have access to drugs like methadone or buprenorphine, many can’t make it to a clinic consistently enough for the treatments to work. Current treatment options also carry the risk of significant side effects and abuse potential.
Atai and DemeRx’s ibogaine treatment intends to be a one-and-done solution for those at the mercy of opioid dependence by activating a neurochemical reset in the brains of those addicted.
In November 2020, Atai announced it had raised $125m in funding to support the preclinical and clinical development for its mental health pipeline, which is grounded in reimagining the uses of so-called “party-drugs” such as MDMA as well as psychedelics like psilocybin.
“This approval allows DemeRx to progress clinical research beyond the previously published uncontrolled studies with ibogaine into well-designed, controlled studies in support of regulatory processes,” said Atai Life Sciences chief scientific officer and co-founder Srinivas Rao.
“With the initiation of this trial, we start a journey towards understanding the potential of DMX-1002, in-line with regulatory bodies. We are optimistic for the future of DMX-1002 in treating OUD – this is a great step forward for DemeRx.”





