
Technology provider Medable has joined forces with the Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard (MRCT Center) to release a toolkit for the decentralised clinical trial (DCT) ethics review process.
The toolkit aims to standardise ethical review and approval and provide a roadmap for the ethical conduct of DCTs. The toolkit can potentially streamline and expedite the institutional review boards (IRBs) and ethics committees (ECs) process by providing a common framework and best practices.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.

“The new DCT IRB/EC Review Toolkit will ease ethics reviews of decentralised trials by establishing commonality and removing some of the guesswork that has made DCT reviews cumbersome,” said Dr Barbara Bierer, professor of medicine at Harvard Medical School and Faculty Director of the MRCT Center.
She added that the guidelines would also inform clinical trial sponsors and investigators on what information should be provided to the IRBs to avoid review delays.
What is in the toolkit?
The toolkit comprises three main sections: people, remote data collection, and data oversight. Each section covers various common elements of DCTs and provides questions for IRBs and ECs to consider.
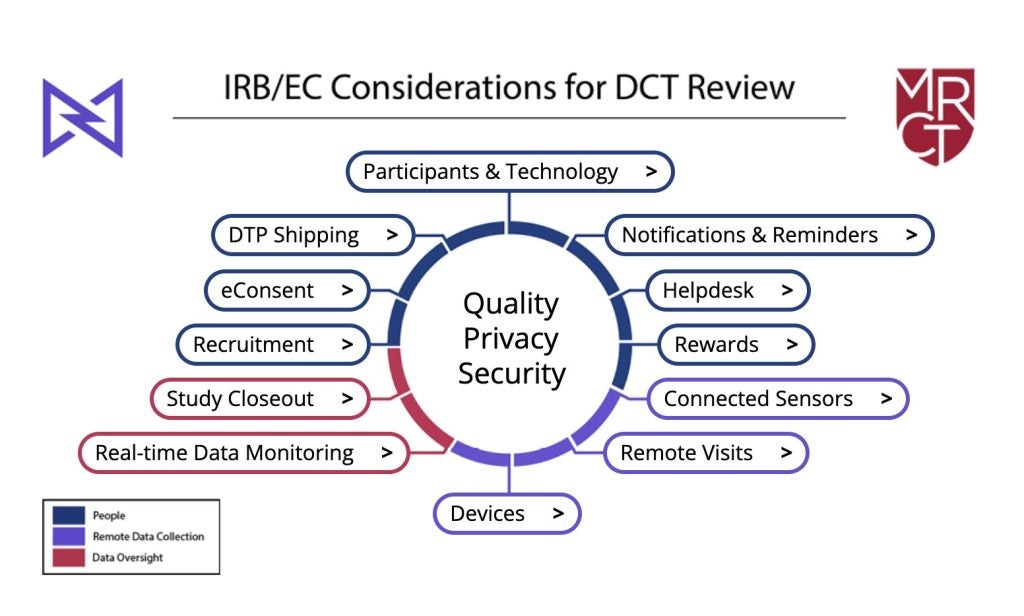
The first section covers topics such as clinical trial recruitment via social media, the use of electronic consent (eConsent), communication with the participants and their preferences, and direct-to-patient shipment of drugs, investigational medical product (IMP), device or sensor.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis section also discusses technology-related topics such as the use of technology and helpdesk functions to troubleshoot software or hardware issues. Finally, the people section looks at rewarding patients for participation.
Clinical Trials Arena spoke with a patient advocate on ethical ways to compensate participants in clinical trials.
The remote data collection section delves into topics such as the inclusion of telemedicine, in-home visits and local providers in the trial protocol, and the potential challenges of these DCT components.
Clinical Trials Arena previously reported on the inclusion of pharmacies to help with patient recruitment. This section of the toolkit also looks at provisioned or patients’ own devices and connected sensors.
Finally, the toolkit explores real-time data monitoring and its impact on patients, as well as the needed steps for clinical trial closeout and documentation.
Medable and MRCT Center partnered with regulatory compliance solution provider Advarra, US Food and Drug Administration (FDA), Office for Human Research Protection (OHRP), ethics committees, sites, patients, and patient advocates to create the DCT toolkit.
Decentralised Clinical Trial coverage in Clinical Trials Arena is supported by Huma.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.





