To understand why sub-Saharan Africa can appear a daunting prospect to a contract research organisation (CRO) or sponsor looking to conduct a clinical trial, you only need to look at a map.
Stretching from the Horn of Africa in the north to the Kalahari Basin in the south and comprised of more than 40 countries – 16 of which are landlocked – its sheer size and make up may appear specifically designed to maximise regulatory and logistical complexity.
However, as much as it appears administratively complex, the operational reality on the ground is rapidly changing. The process of sourcing comparators, ancillaries, and equipment for studies in sub-Saharan Africa has benefited from the development of the region’s pharmaceutical market, with Kenya and its capital city Nairobi developing into a logistics hub that serves as a gateway to countries across the continent.
A comparator is defined as an investigational or marketed drug for reference use in a clinical trial, and with import of these products into Kenya in the first instance, then with the right approvals, they can be exported to other countries in the region where the trials are being carried out.
This gives CROs the opportunity to work with their clinical trial service providers to source either locally or on the international market to ensure they can secure the right drugs in the right locations.
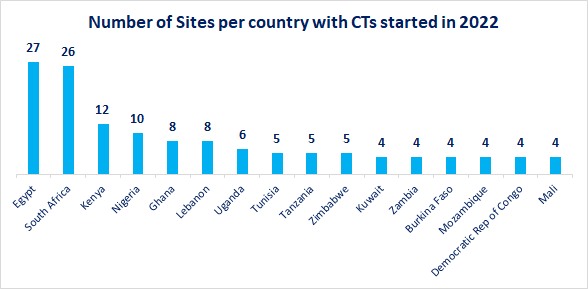
Given that there has been a significant increase in clinical trials conducted in the region, the requirement for proven sourcing strategies is of growing importance. The start of 2022 alone has seen almost double the number of trials in the Middle East and Africa than all other regions combined, with Egypt, South Africa, Kenya, and Nigeria representing the four key sites.
How to reduce transit times
For CRO and sponsors planning studies in sub-Saharan Africa, it is of critical importance that any trial services provider they partner with has secured the appropriate approvals to operate. For example, they must have been verified as an Importer of Record (IoR) for the country where the drugs are first received, such as Kenya, but also have an in depth understanding of the differing rules between countries.
Trial services partners with a deep knowledge of the relevant countries, their local regulations and typical approval times can reduce the timescales to source, import and export comparator products for studies in the region. A process that previously would have taken three to four weeks can be reduced to a matter of days.
“In the case of multi-country trials, all those sites had to access comparators individually and import them individually, whereas now you only have one,” says van den Bergh. “One depot, one source, and one batch.”
Explaining just how significant the differing country regulations and processes can be in influencing the speed and ease of comparator shipments, Rob van den Bergh, sub-Saharan Africa managing director at clinical trial logistics provider Oximio, says: “In Rwanda, it can take three weeks to get an import license but we can do it within 72 hours. In the Democratic Republic of Congo, it can take as long as a month, but we can get an import license in five days.”
The importance of having experts on the ground
This accelerated approval and delivery process is in large part a result of the network of in-country specialists that Oximio has established to understand the unique requirements of the different countries on the continent.
Currently, this includes regulatory experts in more than 10 of the main countries where clinical research is conducted with the plan being to expand it to 20 by the end of the year.
This provides CROs and sponsors with a comparator sourcing process where existing regulatory requirements are factored into their shipments, and that any upcoming changes or new rules are understood and implemented before they can create any additional challenges within the supply chain.
Reducing costs through batch deliveries
In addition to enabling the faster delivery of comparators across sub-Saharan Africa, developing Kenya as a clinical trial logistics hub can also result in cost efficiencies for studies in the region. Reduced transport costs through a storage and distribution scheme, rather than direct-to-site, can reduce the logistics spend per trial.
“You can bulk source the products and store them in a depot, then deliver in batches as and when required to their end destinations, rather than as a single direct-to-country order,” explains Zayheda Khan, commercial and procurement director at Oximio.
This greater flexibility in the distribution of comparators means that shipments be broken down into deliveries to service clinical trials over a longer period but also be delivered to multiple locations in the case of multi-country trials.
“In the case of multi-country trials, all those sites had to access comparators individually and import them individually, whereas now you only have one,” says van den Bergh. “One depot, one source, and one batch.”
To find out more, download the whitepaper below.



