
Empirically valid information documenting the effects of new therapies in children is essential for the safe and successful treatment of pediatric populations – yet robust, age-specific data is often unavailable. Off-label drugs currently make up the majority of medicines used by children, with more than 50% of pediatric medical interventions lacking any randomized controlled trial data. One study even found that for indications where children accounted for nearly 60% of the total disease burden, only 12% of drug trials were focused on pediatric patients.
Children’s prescriptions are usually based on extrapolated data from adults, which can introduce serious risks considering the impact of age on pharmacokinetics and pharmacodynamics. In the US, numerous actions have been taken to increase under-18 representation in trials, including the Pediatric Research Equity Act which authorizes the FDA to require pediatric studies in certain drugs and biological products.
Nevertheless, a large gap remains. The lack of pediatric-only and pediatric-inclusive trials can be attributed to a range of issues, including lack of funding (which typically comes from nonprofit and government organizations), the ethical challenges of conducting research on minors, and the difficulties of recruiting and retaining young patients in studies.
The burden of pediatric trials
The traditional trial model is overly burdensome to children and their caregivers, who must take time off school and work to travel to the clinical site regularly. Many parents might be nervous about the disruption this will bring to their child’s routine, with concerns not only around the length and frequency of study visits but also the number of unpleasant, invasive treatments the child must undergo at the site (e.g. blood draws) and the toll this could take on their physical and emotional wellbeing.
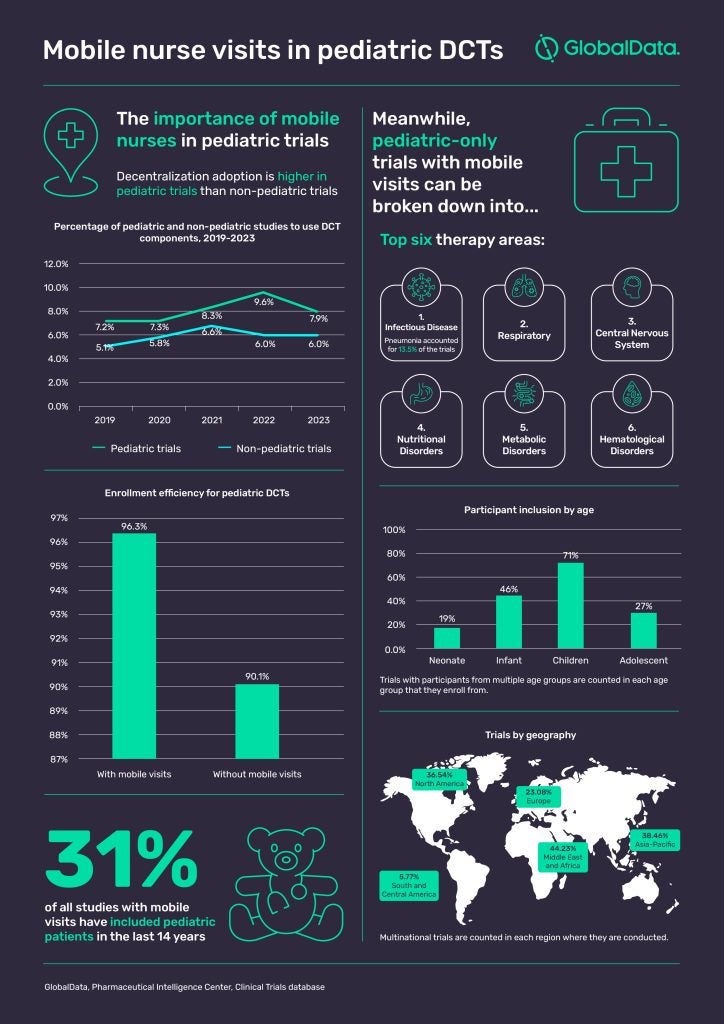
Considering the pool of available participants is already likely to be smaller due to the low prevalence of many childhood diseases, factors like these can have a detrimental impact on participation and retention rates. With parents likely to have some apprehension about their child participating in any form of clinical research, researchers need to consider anything they can do to make it easier for that family to say yes. A growing number of researchers are now seeing the value of the decentralized clinical trial (DCT) model in alleviating the burden of participation. There is increasing hope that decentralization could make trials more child-friendly, and the use of DCTs in pediatric trials has been higher than in non-pediatric trials for years. According to GlobalData’s Clinical Trials database, 9.6% of all pediatric trials in 2022 used at least one element of decentralization such as mobile visits or remote patient monitoring.
Mobile visits – the key to recruiting more children in trials?
DCTs seek to reduce the burden of research participation by lowering or even eliminating the number of visits the child and their caregiver must make into the clinic. They typically combine direct visits from a GCP-trained mobile research nurse with technology elements such as eDiaries and wearables to gather a wealth of data and samples from the patient in a comfortable, non-clinical environment.
Having a mobile research nurse conduct study visits in a convenient, relaxed setting such as the home or school reduces the strain and disruption on the child and their family, including minimizing the time away from class for the child. When pediatric DCTs involve mobile nurses, those visits from a consistent healthcare professional become a constant in the family’s life, helping them to navigate the experience with a trusted adult.
It is no exaggeration to say that the support from a mobile nurse could thus increase the likelihood of that family remaining in the study throughout its duration. Figures from GlobalData’s Clinical Trials database suggest it could even have an impact on enrollment, with pediatric DCTs that included mobile visits achieving an average enrollment efficiency of 96.3% compared to 90.1% for pediatric DCTs without mobile visits.
All of the data on mobile nurse visits point toward pediatric populations being one of their most important use cases. Since 2010, almost a third (31%) of studies with mobile visits have included pediatric patients – more than double the average of 12% – while 17% of studies with mobile visits have focused solely on pediatric patients since 2010.
In the infographic below, we explore the data to understand how and where the industry is making use of mobile nurse services in pediatric-only trials.
Overall, implementing mobile nurse visits in a decentralized pediatric trial is an important strategy for sponsors looking to gather more pediatric data without placing an overly heavy burden on the shoulders of young patients and their families. PCM Trials has led the way in mobile research since 2008, with patients including pediatrics, the elderly, and every group in between. The company’s global network of certified mobile research nurses (CMRNs) – all of whom are registered nurses and many with ICU-level experience – are committed to delivering mobile visits that improve data quality as well as the patient experience, delivering benefits for sponsors, patients, sites, and CROs.
To learn more about how a mobile approach could benefit your trial involving pedatric patients, please download the case study below.


